

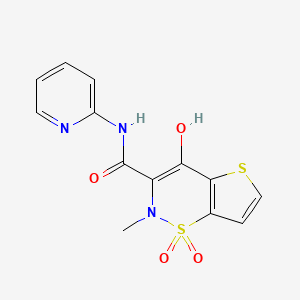

1. 4-hydroxy-2-methyl-n-2-pyridyl-2h-thieno(2,3-e)-1,2-thiazine-3-carboxamide 1,1-dioxide
2. Apo-tenoxicam
3. Artriunic
4. Mobiflex
5. Novo-tenoxicam
6. Reutenox
7. Ro 12-0068
8. Ro-12-0068
9. Tilcotil
1. 59804-37-4
2. Mobiflex
3. Tilcotil
4. Tenoxicamum
5. Liman
6. Tenoxicamum [inn-latin]
7. Ro 12-0068
8. Ro-12-0068
9. Ro 12-0068/000
10. 4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
11. Nsc-758397
12. Mls000069830
13. Chebi:32192
14. Z1r9n0a399
15. 4-hydroxy-2-methyl-n-2-pyridinyl-2h-thieno(2,3-e)-1,2-thiazine-3-carboxamide 1,1-dioxide
16. Ncgc00016889-01
17. Alganex
18. Smr000058865
19. Ro-120068000
20. Cas-59804-37-4
21. 2h-thieno(2,3-e)-1,2-thiazine-3-carboxamide, 4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
22. 2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide,4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
23. 4-hydroxy-2-methyl-1,1-dioxo-n-pyridin-2-ylthieno[2,3-e]thiazine-3-carboxamide
24. Dsstox_cid_25486
25. Dsstox_rid_80909
26. Dsstox_gsid_45486
27. 4-hydroxy-2-methyl-n-2-pyridinyl-2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide
28. Octiveran
29. Rexalgan
30. 4-hydroxy-2-methyl-n-pyridin-2-yl-2h-thieno-[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
31. 4-hydroxy-2-methyl-n-pyridin-2-yl-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
32. Tilcotil (tn)
33. Ccris 5264
34. Sr-01000721894
35. Brn 0572193
36. Tenoxicam (jan/usan/inn)
37. Unii-z1r9n0a399
38. 4-hydroxy-2-methyl-n-2-pyridyl-2h-thieno(2,3-e)-1,2-thiazine-3-carboxamide 1,1-dioxide
39. Tenoxicam [usan:inn:ban:jan]
40. Tenoxicam, Nsaid
41. 2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide, 4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
42. Mobiflex, Tenoxicam
43. Prestwick_849
44. Tenoxicam(mobiflex)
45. Mfcd00083502
46. Ro-120068
47. Spectrum_001430
48. Tenoxicam [inn]
49. Tenoxicam [jan]
50. Tenoxicam [mi]
51. Tenoxicam [usan]
52. Opera_id_1757
53. Prestwick0_000527
54. Prestwick1_000527
55. Prestwick2_000527
56. Prestwick3_000527
57. Spectrum2_001080
58. Spectrum3_001563
59. Spectrum4_000731
60. Spectrum5_001615
61. Tenoxicam [mart.]
62. Tenoxicam [who-dd]
63. (3z)-3-[hydroxy-(pyridin-2-ylamino)methylidene]-2-methyl-1,1-dioxothieno[2,3-e]thiazin-4-one
64. Schembl25343
65. Schembl25344
66. Bspbio_000513
67. Bspbio_003066
68. Kbiogr_001022
69. Kbioss_001910
70. Mls001074071
71. Mls006011432
72. Bidd:gt0650
73. Divk1c_000252
74. Spectrum1503142
75. Spbio_001100
76. Spbio_002434
77. Bpbio1_000565
78. Chembl302795
79. Chembl3188633
80. Dtxsid8045486
81. Schembl13448847
82. Tenoxicam [ep Monograph]
83. Bcbcmap01_000251
84. Bdbm92332
85. Hms500m14
86. Kbio1_000252
87. Kbio2_001910
88. Kbio2_004478
89. Kbio2_007046
90. Kbio3_002566
91. Ninds_000252
92. Hms1569j15
93. Hms1923i15
94. Hms2090p19
95. Hms2092l20
96. Hms2096j15
97. Hms2232k23
98. Hms3370l01
99. Hms3656o10
100. Hms3713j15
101. Hms3884n04
102. Pharmakon1600-01503142
103. Bcp03624
104. Hy-b0440
105. Tox21_110666
106. Bbl028173
107. Ccg-40126
108. Nsc758397
109. S2512
110. Stk637103
111. Zinc12503102
112. Zinc40884328
113. Akos000282084
114. Akos026750148
115. Tenoxicam Degradation Impurity Standard
116. Tox21_110666_1
117. Zinc100006429
118. Ac-4526
119. Db00469
120. Ks-1313
121. Nsc 758397
122. Idi1_000252
123. Smp1_000040
124. Ncgc00016889-02
125. Ncgc00016889-03
126. Ncgc00016889-05
127. Ncgc00016889-06
128. Ncgc00095260-01
129. Ncgc00095260-02
130. Bt166206
131. Bcp0726000170
132. Sbi-0051780.p002
133. Ft-0659572
134. Sw219788-1
135. T2750
136. C75797
137. D01767
138. Q45050
139. Ab00052322-11
140. Ab00052322_12
141. Ab00052322_13
142. 804t374
143. A832479
144. Ro-12-0068/000
145. Sr-01000721894-2
146. Sr-01000721894-4
147. Q63395752
148. Tenoxicam, British Pharmacopoeia (bp) Reference Standard
149. Tenoxicam, European Pharmacopoeia (ep) Reference Standard
150. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide
151. N-(2-pyridyl)-4-hydroxy-2-methyl-2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide
152. (3e)-3-[hydroxy(pyridin-2-ylamino)methylene]-2-methyl-2,3-dihydro4h-thieno[2,3-e] [1,2]thiazin-4-one-1,1-dioxide
153. (3z)-2-methyl-1,1-bis(oxidanylidene)-3-[oxidanyl-(pyridin-2-ylamino)methylidene]thieno[2,3-e][1,2]thiazin-4-one
154. (3z)-3-[hydroxy-(2-pyridinylamino)methylidene]-2-methyl-1,1-dioxo-4-thieno[2,3-e]thiazinone
155. 4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide1,1-dioxide
156. 4-hydroxy-2-methyl-n-pyridin-2-yl-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide (tenoxicam)
| Molecular Weight | 337.4 g/mol |
|---|---|
| Molecular Formula | C13H11N3O4S2 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 337.01909819 g/mol |
| Monoisotopic Mass | 337.01909819 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 599 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of rheumatoid arthritis, osteoarthritis, backache, and pain.
Tenoxicam, an antiinflammatory agent with analgesic and antipyretic properties, is used to treat osteoarthritis and control acute pain.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
M01AC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AC - Oxicams
M01AC02 - Tenoxicam
Absorption
Oral absorption of tenoxicam is rapid and complete (absolute bioavailability 100%).
Tenoxicam is metabolized in the liver to several pharmacologically inactive metabolites (mainly 5'-hydroxy-tenoxicam).
Tenoxicam has known human metabolites that include 5'-Hydroxytenoxicam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
72 hours (range 59 to 74 hours)
The antiinflammatory effects of tenoxicam may result from the inhibition of the enzyme cycooxygenase and the subsequent peripheral inhibition of prostaglandin synthesis. As prostaglandins sensitize pain receptors, their inhibition accounts for the peripheral analgesic effects of tenoxicam. Antipyresis may occur by central action on the hypothalamus, resulting in peripheral dilation, increased cutaneous blood flow, and subsequent heat loss.