

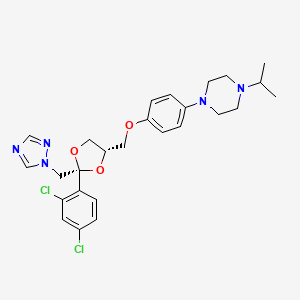

1. 1-(4-((2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-4-(1-methylethyl)piperazine
2. Fungistat
3. Gyno-terazol
4. R 42470
5. Terazol
1. 67915-31-5
2. Triaconazole
3. Terazol 7
4. Gyno-terazol
5. Terazol 3
6. Fungistat
7. 1-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-ylpiperazine
8. Tercospor
9. Chebi:82980
10. Termayazole
11. R 42470
12. R-42470
13. Panlomyc
14. Nsc-331942
15. Ncgc00016912-01
16. Cas-67915-31-5
17. Terazol 3 (tn)
18. (2r,4s)-terconazole
19. Terconazole (usp/inn)
20. Prestwick3_000495
21. Chembl1306
22. Dsstox_cid_25498
23. Dsstox_rid_80917
24. Dsstox_gsid_45498
25. Schembl23165
26. Bspbio_000389
27. Mls002153844
28. Bidd:gt0705
29. Bpbio1_000429
30. Dtxsid2045498
31. Hms2096d11
32. Hms2233c22
33. Hms3713d11
34. Pharmakon1600-01503847
35. Hy-b1790
36. Zinc3873936
37. Tox21_110679
38. Bdbm50375318
39. Mfcd05662369
40. Nsc760361
41. S5033
42. Akos016846147
43. Ccg-213216
44. Cs-6456
45. Db00251
46. Ks-1463
47. Nsc-760361
48. Ncgc00179575-01
49. As-14153
50. Piperazine,1-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-(1-methylethyl)-, Rel-
51. Smr001233206
52. Ab00513849
53. C08080
54. C76152
55. D00888
56. Ab00513849_07
57. 915t315
58. A917374
59. Sr-01000841196
60. J-008500
61. Q7701687
62. Sr-01000841196-2
63. Brd-k86204871-001-02-2
64. Brd-k86204871-001-13-9
65. 1-(4-(((2r,4s)-2-((1h-1,2,4-triazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-4-isopropylpiperazine
66. 1-(4-{[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)-4-(propan-2-yl)piperazine
67. 1-(4-{[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)-4-isopropylpiperazine
68. 1-(4-{[(2r,4s)-2-(2,4-dichlorophenyl)-2-[(1h-1,2,4-triazol-1-yl)methyl]-1,3-dioxolan-4-yl]methoxy}phenyl)-4-(propan-2-yl)piperazine
69. 1-[4-[[(2r)-2alpha-(2,4-dichlorophenyl)-2beta-(1h-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4beta-yl]methoxy]phenyl]-4-(1-methylethyl)piperazine
70. 1-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-yl-piperazine
71. 1h-pyrazole-3-acetamide,5-[[7-[3-[ethyl(2-hydroxyethyl)amino]propoxy]-4-quinazolinyl]amino]-n-(3-fluorophenyl)-
| Molecular Weight | 532.5 g/mol |
|---|---|
| Molecular Formula | C26H31Cl2N5O3 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 531.1803953 g/mol |
| Monoisotopic Mass | 531.1803953 g/mol |
| Topological Polar Surface Area | 64.9 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 693 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Terazol 3 |
| Drug Label | TERAZOL 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-diox... |
| Active Ingredient | Terconazole |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 0.8%; 80mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Terazol 7 |
| PubMed Health | Terconazole (Vaginal) |
| Drug Classes | Antifungal |
| Drug Label | TERAZOL 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-diox... |
| Active Ingredient | Terconazole |
| Dosage Form | Cream |
| Route | Vaginal |
| Strength | 0.4% |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Terconazole |
| PubMed Health | Terconazole (Vaginal) |
| Drug Classes | Antifungal |
| Drug Label | Terconazole vaginal cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1 -ylmethyl)-1,3-dioxolan-4-yl]met... |
| Active Ingredient | Terconazole |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 0.4%; 0.8%; 80mg |
| Market Status | Prescription |
| Company | Nycomed Us; Taro; Fougera Pharms; Perrigo New York |
| 4 of 6 | |
|---|---|
| Drug Name | Terazol 3 |
| Drug Label | TERAZOL 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-diox... |
| Active Ingredient | Terconazole |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 0.8%; 80mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Terazol 7 |
| PubMed Health | Terconazole (Vaginal) |
| Drug Classes | Antifungal |
| Drug Label | TERAZOL 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-diox... |
| Active Ingredient | Terconazole |
| Dosage Form | Cream |
| Route | Vaginal |
| Strength | 0.4% |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Terconazole |
| PubMed Health | Terconazole (Vaginal) |
| Drug Classes | Antifungal |
| Drug Label | Terconazole vaginal cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1 -ylmethyl)-1,3-dioxolan-4-yl]met... |
| Active Ingredient | Terconazole |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 0.4%; 0.8%; 80mg |
| Market Status | Prescription |
| Company | Nycomed Us; Taro; Fougera Pharms; Perrigo New York |
For the treatment of candidiasis (a yeast-like fungal infection) of the vulva and vagina.
FDA Label
Terconazole is a triazole antifungal agent available for intravaginal use. It is structurally related to imidazole-derivative antifungal agents, although terconazole and other triazoles have 3 nitrogens in the azole ring. By inhibiting the 14-alpha-demethylase (lanosterol 14-alpha-demethylase), Terconazole inhibits ergosterol synthesis. Depletion of ergosterol in fungal membrane disrupts the structure and many functions of fungal membrane leading to inhibition of fungal growth.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AG - Triazole derivatives
G01AG02 - Terconazole
Absorption
Following intravaginal administration of terconazole in humans, absorption ranged from 5-8% in three hysterectomized subjects and 12-16% in two non-hysterectomized subjects with tubal ligations
Route of Elimination
Following oral (30 mg) administration of 14C-labelled terconazole, excretion of radioactivity was both by renal (32-56%) and fecal (47-52%) routes.
Systemically absorbed drug appears to be rapidly and extensively metabolized. Terconazole primarily undergoes oxidatative N- and O-dealkylation, dioxolane ring cleavage, and conjugation.
6.9 hours (range 4.0-11.3)
Terconazole may exert its antifungal activity by disrupting normal fungal cell membrane permeability. Terconazole and other triazole antifungal agents inhibit cytochrome P450 14-alpha-demethylase in susceptible fungi, which leads to the accumulation of lanosterol and other methylated sterols and a decrease in ergosterol concentration. Depletion of ergosterol in the membrane disrupts the structure and function of the fungal cell leading to a decrease or inhibition of fungal growth.
