

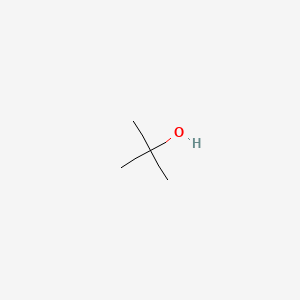

1. Alcohol, Tert-butyl
2. Alcohol, Tertiary-butyl
3. T Butanol
4. T-butanol
5. Tert Butanol
6. Tert Butyl Alcohol
7. Tert-butyl Alcohol
8. Tertiary Butyl Alcohol
9. Tertiary-butyl Alcohol
1. Tert-butyl Alcohol
2. 2-methyl-2-propanol
3. 2-methylpropan-2-ol
4. 75-65-0
5. T-butanol
6. T-butyl Alcohol
7. 1,1-dimethylethanol
8. Trimethylcarbinol
9. T-butyl Hydroxide
10. Trimethyl Carbinol
11. Trimethyl Methanol
12. Tertiary-butyl Alcohol
13. Trimethylmethanol
14. Tert-butylalcohol
15. 2-propanol, 2-methyl-
16. Butanol Tertiaire
17. Dimethylethanol
18. Tertiary Butanol
19. Tertiary-butanol
20. Alcool Butylique Tertiaire
21. Tert- Butyl Alcohol
22. Methanol, Trimethyl-
23. Tertiary Butyl Alcohol
24. Tert.-butanol
25. T-buoh
26. Nci-c55367
27. T-butyl Alchohol
28. Tert-buoh
29. Tert-butyl Hydroxide
30. 2-methyl N-propan-2-ol
31. Md83sfe959
32. Chebi:45895
33. Mfcd00004464
34. Arconol
35. 25-65-0
36. Tert-butyl Alcohol, Anhydrous
37. Caswell No. 124a
38. T-butylalcohol
39. Tert Butanol
40. Tbuoh
41. Butanol Tertiaire [french]
42. Tert Butyl Alcohol
43. Hsdb 50
44. Tbu
45. 2-methylpropanol-2
46. Alcohol, Tert-butyl
47. Ccris 4755
48. Alcool Butylique Tertiaire [french]
49. Einecs 200-889-7
50. Brn 0906698
51. Terbutanol
52. Tertbutanol
53. Tertiarybutanol
54. Unii-md83sfe959
55. Ter-butanol
56. Tert.butanol
57. T-butylalkohol
58. T-butyl-alcohol
59. Terbutyl Alcohol
60. Ai3-01288
61. Tert -butanol
62. Tertbutyl Alcohol
63. Tert. Butanol
64. T- Butanol
65. Hotbu
66. Ter-butyl Alcohol
67. Tert-buyl Alcohol
68. Tert-butyl-alcohol
69. Tert.-butylalcohol
70. Tert.butyl Alcohol
71. Trimethyl-methanol
72. Tert-butanol, Ar
73. 2methyl-2-propanol
74. Tert. Butyl Alcohol
75. Hot-bu
76. Tert.-butyl Alcohol
77. Tert-c4h9oh
78. 2-methyl,2-propanol
79. Methyl-2 Propanol-2
80. T-bu-oh
81. 1,1-dimethyl Ethanol
82. 2-methyl Propan-2-ol
83. 2-methyl-propan-2-ol
84. Tert-butanol Hplc Grade
85. Tert-butanol Acs Reagent
86. Ethanol, 1,1-dimethyl-
87. 2-methyl-2-propyl Alcohol
88. Ec 200-889-7
89. Butyl Alcohol, Tert-
90. (ch3)3coh
91. 4-01-00-01609 (beilstein Handbook Reference)
92. Hoc(ch3)3
93. Chembl16502
94. Tertiary Butyl Alcohol Reagent
95. T-butyl Alcohol [hsdb]
96. T-butyl Alcohol [inci]
97. Dtxsid8020204
98. Tert-butyl Alcohol [ii]
99. Tert-butyl Alcohol [mi]
100. Tert-butanol, >=99% (gc)
101. Tert-butanol, Analytical Standard
102. Tert-butanol, P.a., 99.0%
103. Amy11058
104. Zinc1680021
105. Stl282741
106. Tert-butanol, Anhydrous, >=99.5%
107. Akos009029221
108. Db03900
109. Tert-butanol, For Hplc, >=99.5%
110. Tert-butanol, Technical Grade, 95.0%
111. Tert-butanol 100 Microg/ml In Methanol
112. Tert-butanol, Acs Reagent, >=99.0%
113. Bp-20656
114. Butyl Alcohol (tert)- Reagent Grade Acs
115. Tert-butanol 100 Microg/ml In Acetonitrile
116. Tert-butanol, Saj First Grade, >=98.0%
117. B0706
118. Ft-0688093
119. Tert-butanol, Tebol(r) 99, >=99.3%
120. Tert-butanol, Saj Special Grade, >=99.0%
121. C21389
122. A838477
123. Q285790
124. J-510082
125. F0001-1901
126. Tert-butanol, Puriss. P.a., Acs Reagent, >=99.7% (gc)
127. Tert-butanol, Certified Reference Material, 5000 Mug/ml In Methanol
| Molecular Weight | 74.12 g/mol |
|---|---|
| Molecular Formula | C4H10O |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 74.073164938 g/mol |
| Monoisotopic Mass | 74.073164938 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 25.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/Researchers/ found that t-butyl alcohol is eliminated slowly from the blood of rats. t-Butyl alcohol was dissolved in water and a dose of 25 mmol/kg was administered by gastric intubation to female Wistar rats (number unspecified). The t-butyl alcohol blood concentration at 2 hr was 13.24 mM, at 5 hr it was 12.57 mM, and at 20 hr it was 11.35 mM.
Cosmetic Ingredient Review; Amended Final Report of the Safety Assessment of t-Butyl Alcohol as Used in Cosmetics; International Journal of Toxicology 24 (Suppl 1): 1-20 (2005)
The purpose of this study was to fully characterize the pharmacokinetics of tertiary butyl alcohol in male and female F-344 rats following intravenous administration of 37.5, 75, 150 and 300 mg/kg TBA. TBA was observed to undergo a rapid distribution phase followed by a slower elimination phase. The steady-state volume of distribution for TBA was roughly 4.5 times greater than total body water, and the clearance was lower than the estimated glomerular filtration rate. The elimination of TBA appears to saturate at higher doses, as evidenced by a disproportional increase in area under the concentration-time curve and decreased rate of clearance.
PMID:9334828 Poet TS et al; Toxicol Lett 92 (3): 179-86 (1997)
In animals, tert-butanol is absorbed through the lungs and gastrointestinal tract ... .
WHO/FAO; Environmental Health Criteria Document No. 65: Butanols: Four Isomers (1-Butanol, 2-Butanol, tert-Butanol (1987). Available from, as of November 8, 2013: https://www.inchem.org/pages/ehc.html
t-Butyl alcohol moves rapidly from the blood into the tissues. Eleven male Sprague-Dawley rats were cannulated and intravenously given 350 mg/kg (14)C-t-butyl alcohol. At numerous times following injection, blood samples were withdrawn and the samples measured for radioactivity. There were two phases in the elimination of (14)C-t-butyl alcohol from the blood. The first was a rapid phase, which probably represented the distribution of (14)C-t-butyl alcohol from the blood to other body tissues. The second represented a first-order elimination of radioactivity from the blood with a half-life of approximately 8 hr, indicating that (14)C-t-butyl alcohol was being eliminated primarily as metabolic product(s).
Cosmetic Ingredient Review; Amended Final Report of the Safety Assessment of t-Butyl Alcohol as Used in Cosmetics; International Journal of Toxicology 24 (Suppl 1): 1-20 (2005)
For more Absorption, Distribution and Excretion (Complete) data for T-BUTYL ALCOHOL (9 total), please visit the HSDB record page.
/Researchers/ administered 12 mmol of t-butyl alcohol by stomach tube to three chinchilla rabbits. t-Butyl alcohol was conjugated to a large extent with glucuronic acid, and glucuronides were readily isolated from the rabbit urine; as a percentage of dose, the average extra glucuronic acid excreted over 24 hr was 24.4%. The researchers suggested that volatile alcohols might also be eliminated to some extent in an unchanged state by the lungs. No aldehydes or ketones were detected in the expired air of a rabbit given 6 mL t-butyl alcohol (route unspecified).
Cosmetic Ingredient Review; Amended Final Report of the Safety Assessment of t-Butyl Alcohol as Used in Cosmetics; International Journal of Toxicology 24 (Suppl 1): 1-20 (2005)
t-Butyl alcohol is not a substrate for alcohol dehydrogenase or for the peroxidative activity of catalase, therefore, it is used frequently as an example of a non-metabolizable alcohol. tert-Butyl alcohol is a scavenger of the hydroxyl radical and can be oxidized to formaldehyde and acetone from four different systems; (a) iron catalyzed oxidation of ascorbic acid (b) hydrogen peroxide and iron (c) coupled oxidation of xanthine oxidase, an enzymatic bound system (d) NADPH-dependent microsomal electron transfer, a membrane bound system. Because of its special biochemical properties, t-butyl alcohol may be a valuable probe for the detection of hydroxyl radicals in intact cells and in vivo.
Cederbaum AI et al; Biochem Pharmacol 32 (1983)
In vitro reactions with liver microsomes of mice produced tert-butanol from isobutane.
PMID:3913777 Tsukamoto S et al; J Toxicol Sci 10 (4): 323-32 (1985)
Male Wistar rats exposed to 50, 100, or 300 ppm methyl tertiary-butyl ether vapor ... showed ... blood concns of tert-butanol which were dose dependent indicating metabolic breakdown of the ether in vivo.
PMID:4091653 Salvolainen H et al; Arch Toxicol 57 (4): 285-8 (1985)
For more Metabolism/Metabolites (Complete) data for T-BUTYL ALCOHOL (6 total), please visit the HSDB record page.
Tert-butanol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[(2-methylpropan-2-yl)oxy]oxane-2-carboxylic acid and Tert-butyl hydrogen sulfate.
Tert-butanol is a known human metabolite of tert-butyl ethyl ether (ETBE) and tert-butyl methyl ether.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
In Long-Evans rats treated with tert-butanol (1 g/kg body weight, route not specified), the rate of disappearance of tert- butanol from the blood was apparently of first order with a half life of 9.1 hr.
WHO/FAO; Environmental Health Criteria Document No. 65: Butanols: Four Isomers (1-Butanol, 2-Butanol, tert-Butanol (1987). Available from, as of November 8, 2013: https://www.inchem.org/pages/ehc.html
Two Sprague-Dawley rats were given 1500 mg/kg (14)C-t-butyl alchol by oral gavage. Their blood was sampled at various times following the dosage. ... There was a half-life of 9 hr similar to that seen following intravenous dosing with 350 mg/kg (14)C-t-butyl alchol.
Cosmetic Ingredient Review; Amended Final Report of the Safety Assessment of t-Butyl Alcohol as Used in Cosmetics; International Journal of Toxicology 24 (Suppl 1): 1-20 (2005)
In mice, after a single ip injection of 8.1 mmol tert-butanol/kg body weight, initial blood levels of 8 mmol took 8-9 hr for elimination (blood- tert-butanol half-life was approximately 5 hr). However, after 3 days, inhalation at a vapor concentration to give levels of 8 mmol/L blood, tert-butanol disappeared within 3 hr of removal of mice from the inhalation chamber (half-life of tert- butanol in blood was approximately 1.5 hr).
WHO/FAO; Environmental Health Criteria Document No. 65: Butanols: Four Isomers (1-Butanol, 2-Butanol, tert-Butanol (1987). Available from, as of November 8, 2013: https://www.inchem.org/pages/ehc.html