


1. Paramorphine
2. 115-37-7
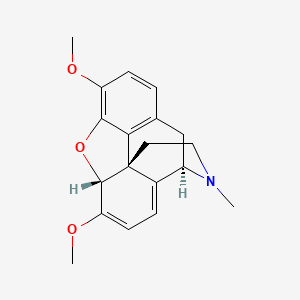
3. Thebain
4. 3-o-methyl-oripavin
5. 4,5alpha-epoxy-3,6-dimethoxy-17-methyl-6,8-morphinadien
6. 2p9mkg8gx7
7. (5r,9r,13s)-4,5-epoxy-3,6-dimethoxy-9alpha-methyl-6,8-morphinadien
8. Chebi:9519
9. (4r,7ar,12bs)-7,9-dimethoxy-3-methyl-2,4,7a,13-tetrahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline
10. Ncgc00160239-01
11. Dsstox_cid_26099
12. Dsstox_rid_81337
13. Dsstox_gsid_46099
14. (5alpha)-6,7,8,14-tetradehydro-4,5-epoxy-3,6-dimethoxy-17-methylmorphinan
15. 3,6-dimethoxy-17-methyl-6,7,8,14-tetradehydro-4,5alpha-epoxymorphinan
16. Cas-115-37-7
17. Einecs 204-084-1
18. Unii-2p9mkg8gx7
19. Tebaine
20. Thenaine
21. Kodein
22. Dea No. 9333
23. Thebaine, Powder
24. Ncgc00247709-01
25. Thebaine [mi]
26. Thebaine [who-dd]
27. Schembl37580
28. Chembl403893
29. Ids-nt-002
30. Dtxsid7046099
31. Schembl19880976
32. Thebaine 0.1 Mg/ml In Methanol
33. Bdbm224032
34. Tox21_111751
35. Tox21_112863
36. Zinc53199482
37. Morphinan, 6,7,8,14-tetradehydro-4,5-alpha-epoxy-3,6-dimethoxy-17-methyl-
38. C06173
39. Codeine Monohydrate Impurity G [ep Impurity]
40. Q63392872
41. Oxycodone Hydrochloride Impurity F [ep Impurity]
42. Thebaine, European Pharmacopoeia (ep) Reference Standard
43. Codeine Hydrochloride Dihydrate Impurity G [ep Impurity]
44. Codeine Phosphate Hemihydrate Impurity G [ep Impurity]
45. Codeine Phosphate Sesquihydrate Impurity G [ep Impurity]
46. 6,7,8,14-tetradehydro-4,5-epoxy-3,6-dimethoxy-17-methylmorphinan
47. Hydrocodone Hydrogen Tartrate 2.5-hydrate Impurity I [ep Impurity]
48. (5.alpha.)-6,7,8,14-tetradehydro-4,5-epoxy-3,6-dimethoxy-17-methylmorphinan
49. Morphinan, 6,7,8,14-tetradehydro-4,5-epoxy-3,6-dimethoxy-17-methyl-,(5.alpha.)-
50. Thebaine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
51. D4r
| Molecular Weight | 311.4 g/mol |
|---|---|
| Molecular Formula | C19H21NO3 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 311.15214353 g/mol |
| Monoisotopic Mass | 311.15214353 g/mol |
| Topological Polar Surface Area | 30.9 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 585 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Convulsants
Substances that act in the brain stem or spinal cord to produce tonic or clonic convulsions, often by removing normal inhibitory tone. They were formerly used to stimulate respiration or as antidotes to barbiturate overdose. They are now most commonly used as experimental tools. (See all compounds classified as Convulsants.)