


1. Ambathizon
2. Amithiozone
3. Conteben
4. Parazone
5. Tb I 698
6. Tb I-698
7. Tb I698
8. Tbi 698
9. Tbi-698
10. Tbi698
11. Thiacetazone
12. Thioacetazon
1. Thiacetazone
2. Amithiozone
3. Ambathizon
4. Benzothiozane
5. Thiocarbazil
6. Conteben
7. Benzothiozon
8. Thioparamizone
9. Benthiozone
10. Thibone
11. Parazone
12. Thioacetazon
13. Tibon
14. Tubin
15. 104-06-3
16. Siocarbazone
17. Tebethione
18. Thiacetone
19. Thiacetozone
20. Thioazetazone
21. Thioparamizon
22. Thiotebesin
23. Thiotebezin
24. Thiotebicina
25. Tiacetazon
26. Tioacetazon
27. Tioatsetazon
28. Tubercazon
29. Aktivan
30. Amitiozon
31. Berkazon
32. Domakol
33. Livazone
34. Mivizon
35. Myvizone
36. Neotibil
37. Neustab
38. Novakol
39. Panrone
40. Seroden
41. Tebalon
42. Tebecure
43. Tebemar
44. Tebethion
45. Tebezon
46. Thiomicid
47. Thionicid
48. Thizone
49. Tibicur
50. Tibizan
51. Tiobicina
52. Tiocarone
53. Tiosecolo
54. Tubigal
55. Antib
56. Diasan
57. Ilbion
58. Thibon
59. Tibone
60. Berculon A
61. Tebesone I
62. 4-acetylaminobenzaldehyde Thiosemicarbazone
63. Nuclon Argentinian
64. Thiosemicarbazone
65. Thiosemicarbarzone
66. Tb I (bayer)
67. 4'-formylacetanilide Thiosemicarbazone
68. Tb I
69. Domagk's T.b.1 Conteben
70. Thioacetazonum
71. Tibione
72. Sdt 1041
73. Magk's T.b.1 Conteben
74. Tb I/698
75. P-acetamidobenzaldehyde Thiosemicarbazone
76. P-acetaminobenzylidenethiosemicarbazone
77. Citazone
78. Tibion
79. Cbc 903150
80. P-formylacetanilide-3-thiosemicarbazone
81. Sq 2321
82. Tb I-698
83. P-acetoaminobenzaldehyde Thiosemicarbazone
84. A 4081
85. 4207 Rp
86. Rp 4207
87. Acetamide, N-[4-[[(aminothioxomethyl)hydrazono]methyl]phenyl]-
88. P-acetylaminobenzadehyde Thiosemicarbazone
89. Nsc-3550
90. Mmg78x7ssr
91. Acetamide, N-(4-(((aminothioxomethyl)hydrazono)methyl)phenyl)-
92. Nsc3550
93. Acetanilide, 4'-formyl-, 4'-(thiosemicarbazone)
94. Thioacetazone (inn)
95. P-acetamidobenzaldehyde Thiosemicarbazon
96. Sq-2321
97. 4-acetamidobenzaldehyde Thiosemicarbazone
98. Ncgc00159389-02
99. Ncgc00159389-03
100. Ncgc00159389-04
101. P-acetylaminobenzaldehyde Thiosemicarbazone
102. N-[4-[(e)-(carbamothioylhydrazinylidene)methyl]phenyl]acetamide
103. Dsstox_cid_2593
104. Thioacetazone [inn]
105. Dsstox_rid_76650
106. Dsstox_gsid_22593
107. N-(4-((2-carbamothioylhydrazono)methyl)phenyl)acetamide
108. Mirizone Neustab
109. Tioacetazona
110. Thioacetazone [inn:ban]
111. Thioacetazonum [inn-latin]
112. Tioacetazona [inn-spanish]
113. Cas-104-06-3
114. Thiosemicarbazone (pharmaceutical)
115. Nsc 3550
116. Einecs 203-170-6
117. Unii-mmg78x7ssr
118. Brn 2810335
119. N-[4-[(e)-(carbamothioylhydrazono)methyl]phenyl]acetamide
120. N-{4-[(e)-(carbamothioylhydrazono)methyl]phenyl}acetamide
121. Acetamide, N-(4-(((aminothiomethyl)hydrazono)methylene)phenyl)-
122. Acetamide, N-[4-[[(aminothiomethyl)hydrazono]methylene]phenyl]-
123. Ai3-18591
124. 4207rp
125. Citazone (tn)
126. Acetanilide, 4'-formyl-, Thiosemicarbazone
127. Thiacetazone [mi]
128. Schembl42515
129. Acetanilide, Thiosemicarbazone
130. Wln: Suyzmnu1r Dmv1
131. 4-14-00-00075 (beilstein Handbook Reference)
132. N-(4-([2-(aminocarbothioyl)hydrazono]methyl)phenyl)acetamide
133. Thioacetazone [mart.]
134. Acetamide, N1-(4-([2-(aminocarbothioyl)hydrazono]methyl)phenyl)
135. Thioacetazone [who-dd]
136. Thioacetazone [who-ip]
137. Chembl375492
138. Tb-1
139. Acetamide, N-[4-[[2-(aminothioxomethyl)hydrazinylidene]methyl]phenyl]-
140. Dtxsid80859179
141. Ex-a101
142. Chebi:134958
143. Hy-b1526
144. Tox21_111625
145. Tox21_111626
146. Acetanilide, 4'-(thiosemicarbazone)
147. Bdbm50247903
148. Mfcd00022157
149. Rp4207
150. Sq2321
151. Stl503688
152. Zinc32709513
153. Thioacetazonum [who-ip Latin]
154. Akos000304458
155. Tox21_111625_1
156. Db12829
157. Ac-36791
158. As-71466
159. Cs-0013329
160. D08584
161. A800888
162. Sr-01000872620
163. Sr-01000872620-2
164. N-{4-[(e)-[(carbamothioylamino)imino]methyl]phenyl}acetamide
165. N-(4-{(e)-[(aminocarbonothioyl)hydrazono]methyl}phenyl)acetamide
166. N-{4-[(e)-(2-carbamothioylhydrazinylidene)methyl]phenyl}acetamide
167. N-(4-(((amino-thioxomethyl)hydrazono)methyl)phenyl)acetamide [who-ip]
168. 910379-02-1
169. N-[4-[(2-carbamothioylhydrazono)methyl]phenyl]acetamide;n-[4-[(e)-(carbamothioylhydrazono)methyl]phenyl]acetamide
| Molecular Weight | 236.30 g/mol |
|---|---|
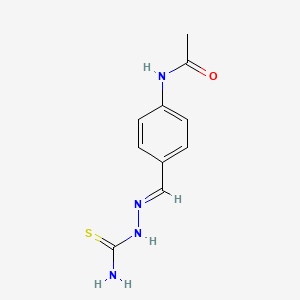
| Molecular Formula | C10H12N4OS |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 236.07318219 g/mol |
| Monoisotopic Mass | 236.07318219 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 285 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AK - Other drugs for treatment of tuberculosis
J04AK07 - Thioacetazone
