
1. Aldazine
2. Apo Thioridazine
3. Apo-thioridazine
4. Apothioridazine
5. Meleril
6. Mellaril
7. Melleretten
8. Melleril
9. Melleryl
10. Melzine
11. Rideril
12. Sonapax
13. Thioridazine Hcl
14. Thioridazine Hydrochloride
15. Thioridazine Neurazpharm
16. Thioridazine-neurazpharm
17. Thioridazineneurazpharm
18. Thiozine
1. 50-52-2
2. Mellaril
3. Thioridazin
4. Sonapax
5. Mellerette
6. Melleril
7. Meleril
8. Mellaril-s
9. Malloryl
10. Dl-thioridazine
11. Mallorol
12. Mellerets
13. Melleretten
14. Tioridazina
15. Tp-21
16. Thioridazine Prolongatum
17. Melleril (liquid)
18. Novoridazine
19. Thioridazinum
20. 10-(2-(1-methylpiperidin-2-yl)ethyl)-2-(methylthio)-10h-phenothiazine
21. Orsanil
22. Thioril
23. 2-methylmercapto-10-(2-(n-methyl-2-piperidyl)ethyl)phenothiazine
24. 10-(2-(1-methyl-2-piperidyl)ethyl)-2-(methylthio)phenothiazine
25. 3-methylmercapto-n-(2'-(n-methyl-2-piperidyl)ethyl)phenothiazine
26. 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine
27. 10h-phenothiazine, 10-(2-(1-methyl-2-piperidinyl)ethyl)-2-(methylthio)-
28. Melleryl
29. Chembl479
30. 10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylsulfanyl-phenothiazine
31. N3d6tg58ni
32. 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-(methylsulfanyl)-10h-phenothiazine
33. Chebi:9566
34. Ncgc00016059-05
35. (+-)-thioridazine
36. Mellarit
37. Thiozine
38. Malloryl; Meleril; Mellaril S; Mellerets
39. Phenothiazine, 10-((1-methyl-2-piperidyl)ethyl)-2-(methylthio)-
40. Dsstox_cid_3656
41. 10h-phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-
42. Dsstox_rid_77130
43. Dsstox_gsid_23656
44. Thioridazinum [inn-latin]
45. Tioridazina [inn-spanish]
46. Thioxidazine
47. Thioridazine, Prolongatum
48. Phenothiazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylthio)-
49. 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-(methylthio)-10h-phenothiazine
50. Cas-50-52-2
51. Mellaril-s (tn)
52. Nsc186060
53. Hsdb 3189
54. Einecs 200-044-2
55. Thioridazine (usp/inn)
56. Unii-n3d6tg58ni
57. Mellaril (*hydrochloride*)
58. Ai3-51923
59. Thioridazine [usan:usp:inn:ban]
60. Mellaril (salt/mix)
61. Spectrum_001066
62. Prestwick0_000078
63. Prestwick1_000078
64. Prestwick2_000078
65. Prestwick3_000078
66. Spectrum2_001332
67. Spectrum3_000585
68. Spectrum4_000356
69. Spectrum5_001062
70. Thioridazine [mi]
71. (.+/-.)-thioridazine
72. Thioridazine [inn]
73. Biomol-nt_000017
74. Thioridazine [hsdb]
75. Thioridazine [usan]
76. Schembl9566
77. Thioridazine [vandf]
78. Lopac0_001252
79. Bspbio_000295
80. Bspbio_002030
81. Gtpl100
82. Kbiogr_000791
83. Kbiogr_002308
84. Kbioss_001546
85. Kbioss_002310
86. Thioridazine [mart.]
87. (+/-)-thioridazine
88. Divk1c_000066
89. Thioridazine [usp-rs]
90. Thioridazine [who-dd]
91. Spbio_001483
92. Spbio_002216
93. Bpbio1_000325
94. Bpbio1_001175
95. Dtxsid6023656
96. Schembl12434241
97. Hy-b0965a
98. Kbio1_000066
99. Kbio2_001546
100. Kbio2_002308
101. Kbio2_004114
102. Kbio2_004876
103. Kbio2_006682
104. Kbio2_007444
105. Kbio3_001530
106. Kbio3_002788
107. Cmap_000015
108. Ninds_000066
109. Hms2090j04
110. Thioridazine [orange Book]
111. Thioridazine [ep Monograph]
112. Bcp19922
113. Thioridazine [usp Monograph]
114. Tox21_110294
115. Bdbm50002338
116. Ns-835
117. 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylsulfanyl)-10h-phenothiazine
118. Tox21_110294_1
119. Ccg-205326
120. Db00679
121. Sdccgsbi-0051219.p005
122. Idi1_000066
123. Ncgc00016059-03
124. Ncgc00016059-04
125. Ncgc00016059-07
126. Ncgc00016059-08
127. Ncgc00016059-09
128. Ncgc00016059-10
129. Ncgc00016059-11
130. Ncgc00016059-13
131. Ncgc00016059-14
132. Ncgc00016059-24
133. Ncgc00089809-02
134. Ncgc00089809-03
135. As-77485
136. Sbi-0051219.p004
137. Ab00053553
138. Cs-0013830
139. D00373
140. D94995
141. Q58375
142. Ab00053553-16
143. Ab00053553_17
144. Ab00053553_18
145. 242t875
146. L001321
147. Brd-a84481105-003-17-2
148. 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-10h-phenothiazine
149. 10-[2-(1-methyl-piperidin-2-yl)-ethyl]-2-methylsulfanyl-10h-phenothiazine
150. 10h-phenothiazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylthio-
151. (thioridazine)10-[2-(1-methyl-piperidin-2-yl)-ethyl]-2-methylsulfanyl-10h-phenothiazine
152. 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylsulfanyl)-10h-phenothiazine #
153. 10-[2-(1-methyl-piperidin-2-yl)-ethyl]-2-methylsulfanyl-10h-phenothiazine (thioridazine)
154. Thioridazine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
155. 107388-89-6
| Molecular Weight | 370.6 g/mol |
|---|---|
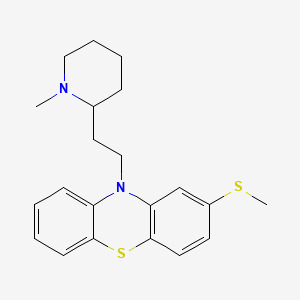
| Molecular Formula | C21H26N2S2 |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 370.15374118 g/mol |
| Monoisotopic Mass | 370.15374118 g/mol |
| Topological Polar Surface Area | 57.1 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 432 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsychotic Agents, Phenothiazine; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Thioridazine is indicated for the management of schizophrenic patients who fail to respond adequately to treatment with other antipsychotic drugs. Due to the risk of significant, potentially life threatening, proarrhythmic effects with thioridazine treatment, thioridazine should be used only in patients who have failed to respond adequately to treatment with appropriate courses of other antipsychotic drugs, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs. Consequently, before initiating treatment with thioridazine, it is strongly recommended that a patient be given at least two trials, each with a different antipsychotic drug product, at an adequate dose, and for an adequate duration. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Thioridazine Hydrochloride tablet, film coated (February 2010). Available from, as of June 28, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16596
The prescriber should be aware that thioridazine has not been systematically evaluated in controlled trials in treatment refractory schizophrenic patients and its efficacy in such patients is unknown. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Thioridazine Hydrochloride tablet, film coated (February 2010). Available from, as of June 28, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16596
The US Food and Drug Administration (FDA) currently advises clinicians that antipsychotic agents are not approved for the treatment of dementia-related psychosis. FDA further advises clinicians that no drugs currently are approved for the treatment of patients with dementia-associated psychosis and that other management options should be considered in such patients. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2509
... Extrapyramidal reactions ... fairly common, usually 3 types ... Parkinsonian-like syndrome ... dystonia and dyskinesia, including torticollis, tics, and other involuntary muscle movements ... akathisia, shown by restlessness ... hyperreflexia, reported in newborn ... ./Phenothiazines/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1021
Thioridazine has been shown to prolong the QTc interval in a dose related manner, and drugs with this potential, including thioridazine, have been associated with Torsades de pointes type arrhythmias and sudden death. Due to its potential for significant, possibly life threatening, proarrhythmic effects, thioridazine should be reserved for use in the treatment of schizophrenic patients who fail to show an acceptable response to adequate courses of treatment with other antipsychotic drugs, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs
US Natl Inst Health; DailyMed. Current Medication Information for Thioridazine Hydrochloride tablet, film coated (February 2010). Available from, as of June 28, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16596
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thioridazine hydrochloride is not approved for the treatment of patients with dementia-related psychosis
US Natl Inst Health; DailyMed. Current Medication Information for Thioridazine Hydrochloride tablet, film coated (February 2010). Available from, as of June 28, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16596
In common with other phenothiazines, thioridazine is contraindicated in severe central nervous system depression or comatose states from any cause including drug induced central nervous system depression. It should also be noted that hypertensive or hypotensive heart disease of extreme degree is a contraindication of phenothiazine administration.
US Natl Inst Health; DailyMed. Current Medication Information for Thioridazine Hydrochloride tablet, film coated (February 2010). Available from, as of June 28, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16596
For more Drug Warnings (Complete) data for Thioridazine (48 total), please visit the HSDB record page.
For the treatment of schizophrenia and generalized anxiety disorder.
Thioridazine is a trifluoro-methyl phenothiazine derivative intended for the management of schizophrenia and other psychotic disorders. Thioridazine has not been shown effective in the management of behaviorial complications in patients with mental retardation.
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AC - Phenothiazines with piperidine structure
N05AC02 - Thioridazine
Absorption
60%
Experimental studies in animals and in vitro have demonstrated that thioridazine has affinity for melanin granules and tends to accumulate in close association with uveal pigment ... .
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 908
... Pharmacokinetics and metabolism ... similar ... to chlorpromazine, but strong anticholinergic action of thioridazine on the gut may modify its own absorption ... . Concentrations of thioridazine in plasma are relatively high (hundreds of nanograms per milliliter), possibly owing to its relative hydrophilicity ... .
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 395
In 48 patients taking thiordazine the mean amount not bound to serum proteins was 0.15%, that of the side-chain sulfoxide 1.66%, side-chain sulfone 1.17%, and ring sulfoxide 1.7%.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1560
Thioridazine and metabolites were measured in brain, liver, and kidney specimens, obtained postmortem from two subjects whose deaths were related to acute intoxication with thioridazine, by gas-liquid chromatography. Although the absolute concentration measured for thioridazine and metabolites differed in the two cases, the metabolic pattern for each tissue, expressed in terms of the percentage of total drug in each tissue, was quite similar. The brain, liver, and kidney metabolic patterns, however, are in sharp contrast to the plasma metabolite patterns observed for subjects on a therapeutic regimen of thioridazine. As this example demonstrates, postmortem specimens are a valuable (but seldom used) source of human pharmacological data.
PMID:699294 Dinovo E et al; Clin Chem 24 (10): 1828-30 (1978)
For more Absorption, Distribution and Excretion (Complete) data for Thioridazine (10 total), please visit the HSDB record page.
Hepatic
Major metabolites include sulfoxy products at ring position 5 (inactive) or at substituent at position 2 (including active metabolite mesoridazine). Demethylation of piperidine ring is very rapid ... .
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 395
Although the exact metabolic fate of phenothiazines has not been clearly established, the drugs are extensively metabolized, principally in the liver via hydroxylation, oxidation, demethylation, sulfoxide formation, and conjugation with glucuronic acid; metabolic alterations in the side chain also may occur. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511
Thioridazine and metabolites were determined by a selective HPLC technique in blood from five post-mortem cases; two deaths attributed to drug overdose and three deaths due to natural causes or trauma. Additionally, total thioridazine-like compounds were determined in these blood samples and liver specimens by a nonspecific fluorometric technique. Blood concentrations were: thioridazine, 0.78-8.85 mg/L; mesoridazine, 0.52-26.8 mg/L; and sulforidazine, 0.00-0.87 mg/L. Thioridazine-5-sulfoxide stereoisomeric DL,LD, and DD,LL pair concentrations ranged from 0.02-0.56 and 0.03-0.83 mg/L, respectively. Thioridazine metabolite profiles were not helpful in differentiating therapeutic administration from severe overdose. Liver appears to be the specimen of choice in the assessment of thioridazine overdose.
PMID:7176556 Poklis A et al; J Anal Toxicol 6 (5): 250-2 (1982)
For more Metabolism/Metabolites (Complete) data for Thioridazine (8 total), please visit the HSDB record page.
Thioridazine has known human metabolites that include N-desmethylthioridazine, Thioridazine 2-sulfoxide, and Thioridazine 5-sulfoxide.
Sulphoridazine is a known human metabolite of schembl149458.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
21-25 hours
Serum half-life of thioridazine has been estimated to range from about 6 to over 40 hours.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 1560
Thioridazine blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; blocks alpha-adrenergic effect, depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis.
The basic pharmacological activity of thioridazine is similar to that of other phenothiazines, but is associated with minimal extrapyramidal stimulation.
US Natl Inst Health; DailyMed. Current Medication Information for Thioridazine Hydrochloride tablet, film coated (February 2010). Available from, as of June 28, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=16596
The principal pharmacologic effects of thioridazine are similar to those of chlorpromazine. On a weight basis, thioridazine is about as potent as chlorpromazine. Thioridazine has strong anticholinergic and sedative effects and weak extrapyramidal effects. Thioridazine has little antiemetic activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010)
The development of phenothiazine derivatives as psychopharmacologic agents resulted from the observation that certain phenothiazine antihistaminic compounds produced sedation. In an attempt to enhance the sedative effects of these drugs, promethazine and chlorpromazine were synthesized. Chlorpromazine is the pharmacologic prototype of the phenothiazines. The pharmacology of phenothiazines is complex, and because of their actions on the central and autonomic nervous systems, the drugs affect many different sites in the body. Although the actions of the various phenothiazines are generally similar, these drugs differ both quantitatively and qualitatively in the extent to which they produce specific pharmacologic effects. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2510
In the CNS, phenothiazines act principally at the subcortical levels of the reticular formation, limbic system, and hypothalamus. Phenothiazines generally do not produce substantial cortical depression; however, there is minimal information on the specific effects of phenothiazines at the cortical level. Phenothiazines also act in the basal ganglia, exhibiting extrapyramidal effects. The precise mechanism(s) of action, including antipsychotic action, of phenothiazines has not been determined, but may be principally related to antidopaminergic effects of the drugs. There is evidence to indicate that phenothiazines antagonize dopamine-mediated neurotransmission at the synapses. There is also some evidence that phenothiazines may block postsynaptic dopamine receptor sites. However, it has not been determined whether the antipsychotic effect of the drugs is causally related to their antidopaminergic effects. Phenothiazines also have peripheral and/or central antagonistic activity against alpha-adrenergic, serotonergic, histaminic (H1-receptors), and muscarinic receptors. Phenothiazines also have some adrenergic activity, since they block the reuptake of monoamines at the presynaptic neuronal membrane, which tends to enhance neurotransmission. The effects of phenothiazines on the autonomic nervous system are complex and unpredictable because the drugs exhibit varying degrees of alpha-adrenergic blocking, muscarinic blocking, and adrenergic activity. The antipsychotic activity of phenothiazines may be related to any or all of these effects, but it has been suggested that the drugs' effects on dopamine are probably most important. It has also been suggested that effects of phenothiazines on other amines (eg, gamma-aminobutyric acid [GABA]) or peptides (eg, substance P, endorphins) may contribute to their antipsychotic effect. Further study is needed to determine the role of central neuronal receptor antagonism and of effects on biochemical mediators in the antipsychotic action of the phenothiazines and other antipsychotic agents. /Phenothiazine General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2510
For more Mechanism of Action (Complete) data for Thioridazine (13 total), please visit the HSDB record page.