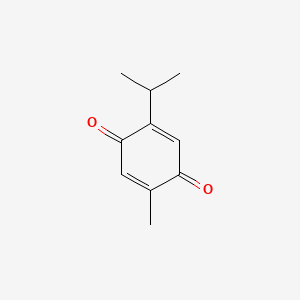

1. 2-isopropyl-5-methylbenzoquinone
2. 2-methyl-5-isopropyl-p-benzoquinone
3. Dihydrothymoquinone
1. 490-91-5
2. Thymoquinon
3. P-cymene-2,5-dione
4. 2-isopropyl-5-methyl-1,4-benzoquinone
5. P-mentha-3,6-diene-2,5-dione
6. 2,5-cyclohexadiene-1,4-dione, 2-methyl-5-(1-methylethyl)-
7. 5-isopropyl-2-methyl-1,4-benzoquinone
8. 2-isopropyl-5-methyl-p-benzoquinone
9. 2-isopropyl-5-methylbenzoquinone
10. 2-isopropyl-5-methylbenzo-1,4-quinone
11. 2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione
12. Nsc 2228
13. 2-isopropyl-5-methylcyclohexa-2,5-diene-1,4-dione
14. 2-methyl-5-isopropyl-p-benzoquinone
15. Nsc2228
16. 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione
17. Nsc-2228
18. 2-methyl-5-isopropyl-1,4-benzoquinone
19. O60ie26nuf
20. 2,5-cyclohexadiene-1,4-dione, 5-isopropyl-2-methyl-
21. 5-isopropyl-2-methyl-p-benzoquinone
22. 2-methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione
23. 5-isopropyl-2-methyl-2,5-cyclohexadiene-1,4-dione
24. Polythymoquinone
25. Ccris 7152
26. Einecs 207-721-1
27. Brn 1939047
28. Thymolquinone
29. Thymoil
30. Ai3-17758
31. 4hco
32. Mfcd00001602
33. P-mentha-3,5-dione
34. Spectrum_001237
35. Specplus_000457
36. Thymoquinone, >=98%
37. Spectrum2_000700
38. Spectrum3_001345
39. Spectrum4_001895
40. Spectrum5_000550
41. Unii-o60ie26nuf
42. Bspbio_003129
43. Kbiogr_002455
44. Kbioss_001717
45. Divk1c_006553
46. Schembl542535
47. Thymoquinone [who-dd]
48. Spbio_000859
49. Chembl1672002
50. Dtxsid9060079
51. Kbio1_001497
52. Kbio2_001717
53. Kbio2_004285
54. Kbio2_006853
55. Kbio3_002349
56. Thymoquinone, Analytical Standard
57. Chebi:113532
58. 2-methyl-5-iso-propylbenzoquinone
59. Bdbm166686
60. Zinc164367
61. Bcp16946
62. Hy-d0803
63. Wln: L6v Dvj B1 Ey1&1
64. 2,4-dione, 5-isopropyl-2-methyl-
65. Ccg-40027
66. S4761
67. Akos003368628
68. Ncgc00178250-01
69. Ncgc00178250-05
70. As-11327
71. P-mentha-3,6-diene-2,5-dione (8ci)
72. 2-isopropyl-5-methylbenzo-1,4-quinone #
73. 2,4-dione, 2-methyl-5-(1-methylethyl)-
74. Cs-0012226
75. Ft-0612708
76. T0795
77. T72517
78. A827656
79. Sr-05000002192
80. Q7799650
81. Sr-05000002192-2
82. W-202869
83. Brd-k97566842-001-03-5
84. Thymoquinone; 2-isopropyl-5-methylbenzo-1,4-quinone
85. 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione (f8)
86. 2-methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione, 9ci
87. Ethyl 2-hydroxy-5-[[2-(trifluoromethyl)phenyl]carbamoyl]benzoate;thymoquinon
| Molecular Weight | 164.20 g/mol |
|---|---|
| Molecular Formula | C10H12O2 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 164.083729621 g/mol |
| Monoisotopic Mass | 164.083729621 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 293 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Thymoquinone is a natural compound with widespread protective effects, including anti-oxidative, anti-inflammatory, immunomodulatory, anti-cancer, and anti-microbial. It is able to induce apoptosis, regulate pro- and anti- apopotitic genes, and inhibit cancer metastasis through JNK and p38 activation. Its anti-inflammatory effects are attributed to its inhibition of inflammatory cytokines and processes, including pathways associated with 5-LO, COX, and PGD2.