


1. Fk 1160
2. Nta-194
3. Solantal
4. Tiaramide
5. Tiaramide Monohydrochloride
1. 35941-71-0
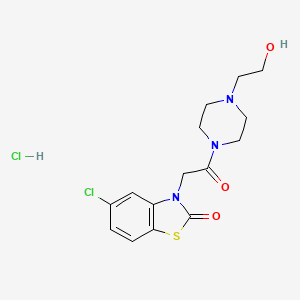
2. 5-chloro-3-(2-(4-(2-hydroxyethyl)piperazin-1-yl)-2-oxoethyl)benzo[d]thiazol-2(3h)-one Hydrochloride
3. Tiaramide Hcl
4. Solantal
5. Nta-194
6. Tiaramide Hydrochloride-r
7. Usv 2592 Hcl
8. Fk 1160
9. Nsc-289337
10. Fk-1160
11. 4-[(5-chloro-2-oxo-2h-benzothiazol-3-yl)acetyl]piperazine-1-ethanol Monohydrochloride
12. Ity1616x9t
13. Rhc-2592
14. 35941-71-0 (hcl)
15. 4-((5-chloro-2-oxo-3-benzothiazolinyl)acetyl)-1-piperazineethanol Monohydrochloride
16. Thiaramide
17. Fk 1160; Nsc 289337; Nta 194; Rhc 2592
18. Ventaval Tabletten
19. 1-piperazineethanol, 4-((5-chloro-2-oxo-3(2h)-benzothiazolyl)acetyl)-, Monohydrochloride
20. Thiaramide [german]
21. Nsc289337
22. Unii-ity1616x9t
23. 4-((5-chloro-2-oxo-2h-benzothiazol-3-yl)acetyl)piperazine-1-ethanol Monohydrochloride
24. Nta 194
25. Solantal (tn)
26. Einecs 252-802-7
27. Tiaramide Hydrochloride [usan:jan]
28. Nta-194tiaramide
29. Nsc 289337
30. Dsstox_cid_28683
31. Dsstox_rid_82953
32. Nta-194 Hcl
33. Dsstox_gsid_48757
34. Schembl181916
35. Chembl538059
36. Dtxsid6048757
37. Chebi:32222
38. Tiaramide Hydrochloride [mi]
39. Tox21_113221
40. Bdbm50222213
41. Fk1160
42. Mfcd01702832
43. Tiaramide Hydrochloride [jan]
44. 1-piperazineethanol, Monohydrochloride
45. Tiaramide Hydrochloride [usan]
46. Akos024464353
47. Tiaramide Hydrochloride (jp17/usan)
48. Tiaramide Hydrochloride [mart.]
49. 2-benzothiazolinone, 5-chloro-3-(4-(2-hydroxyethyl)-1-piperazinyl)carbonylmethyl-, Hcl
50. 4-((5-chloro-2-oxo-3(2h)-benzothiazolyl)acetyl)-1-piperazineethanol Hydrochloride
51. 5-chloro-3-(4-(2-hydroxyethyl)-1-piperazinyl)carbonylmethyl-2-benzothiazolinone Hydrochloride
52. Tiaramide Hydrochloride [who-dd]
53. 5-chloro-3-[2-[4-(2-hydroxyethyl)piperazin-1-yl]-2-oxoethyl]-1,3-benzothiazol-2-one;hydrochloride
54. As-74709
55. Cas-35941-71-0
56. T3176
57. D01341
58. D92667
59. A874495
60. Q27280902
61. 1-piperazineethanol, 4-((5-chloro-2-oxo-3(2h)-benzothiazolyl)acetyl)-, Hydrochloride
62. 4-[(5-chloro-2-oxo-2h-benzothiazol-3-yl)acetyl]piperazine-1-ethanolmonohydrochloride
63. 4-[(5-chloro-2-oxo-3-benzothiazolinyl)acetyl]-1-piperazineethanol Hydrochloride
64. 2(3h)-benzothiazolone, 5-chloro-3-(2-(4-(2-hydroxyethyl)-1-piperazinyl)-2-oxoethyl)-, Hydrochloride (1:1)
65. 5-chloro-3-(2-(4-(2-hydroxyethyl)piperazin-1-yl)-2-oxoethyl)benzo[d]thiazol-2(3h)-onehydrochloride
66. 5-chloro-3-[[4-(2-hydroxyethyl)piperazin-1-yl]carbonylmethyl]-2-benzothiazolinone Hydrochloride
67. 5-chloro-3-{2-[4-(2-hydroxyethyl)piperazin-1-yl]-2-oxoethyl}-2,3-dihydro-1,3-benzothiazol-2-one Hydrochloride
| Molecular Weight | 392.3 g/mol |
|---|---|
| Molecular Formula | C15H19Cl2N3O3S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 391.0524180 g/mol |
| Monoisotopic Mass | 391.0524180 g/mol |
| Topological Polar Surface Area | 89.4 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 459 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)