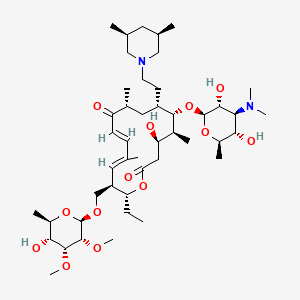

1. 20-deoxo-20-(3,5-dimethylpiperidin-1-yl)desmycosin
2. El 870
3. El-870
4. Micotil 300
1. 108050-54-0
2. Micotil 300
3. El-870
4. El870
5. Ly177370
6. Ly-177370
7. Xl4103x2e3
8. Nsc-759584
9. Tilmicosin 100 Microg/ml In Acetonitrile
10. Tilmicosina
11. Tilmicosine
12. Tilmicosinum
13. Tilmicosine [inn-french]
14. Tilmicosinum [inn-latin]
15. Tilmicosina [inn-spanish]
16. Hsdb 7446
17. Unii-xl4103x2e3
18. Tilmicosin [usan:usp:inn:ban]
19. Ncgc00096003-01
20. (4r,5s,6s,7r,9r,11e,13e,15r,16r)-6-[(2r,3r,4s,5s,6r)-4-(dimethylamino)-3,5-dihydroxy-6-methyloxan-2-yl]oxy-7-[2-[(3s,5r)-3,5-dimethylpiperidin-1-yl]ethyl]-16-ethyl-4-hydroxy-15-[[(2r,3r,4r,5r,6r)-5-hy
21. Droxy-3,4-dimethoxy-6-methyloxan-2-yl]oxymethyl]-5,9,13-trimethyl-1-oxacyclohexadeca-11,13-diene-2,10-dione
22. Ly-177370;el-870
23. Micotil (tn)
24. Mfcd00864842
25. Tilmicosin [mi]
26. Tilmicosin (usp/inn)
27. Tilmicosin [inn]
28. Tilmicosin [hsdb]
29. Tilmicosin [usan]
30. Tilmicosin [mart.]
31. Dsstox_cid_26011
32. Dsstox_rid_81287
33. Tilmicosin [usp-rs]
34. Tilmicosin [who-dd]
35. Dsstox_gsid_46011
36. 4(sup A)-o-de(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-20-deoxo-20-(cis-3,5-dimethylpiperidino)tylosin
37. Schembl149192
38. Tilmicosin [green Book]
39. Chembl1908333
40. Dtxsid5046011
41. Tilmicosin [usp Monograph]
42. Act06683
43. Hy-b0905
44. Tox21_111546
45. S4122
46. Tilmicosin 100 Microg/ml In Methanol
47. Zinc238809114
48. Zinc245204941
49. Ccg-270545
50. Db11471
51. Nsc 759584
52. Tilmicosin 1000 Microg/ml In Methanol
53. Ncgc00348375-01
54. Ncgc00348375-02
55. Tylosin, 4(sup A)-o-de(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-20-deoxo-20-(3,5-dimethyl-1-piperidinyl)-, 20(cis)-
56. Tylosin, 4a-o-de(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-20-deoxo-20-(3,5-dimethyl-1-piperidinyl)-
57. Cas-108050-54-0
58. D02492
59. Ab01566912_01
60. 050t540
61. Q722387
62. Tilmicosine, Antibiotic For Culture Media Use Only
63. Q-100992
64. 4 Sup(a)-o-de(2,6-dideoxy-3-c-methyl-.alpha.-l-ribo-hexopyranosyl)-20-deoxo-20-(cis-3,5-dimethylpiperidino)tylosin
65. Tylosin, 4 Sup(a)-o-de(2,6-dideoxy-3-c-methyl-.alpha.-l-ribo-hexopyranosyl)-20-deoxo-20-(3,5-dimethyl-1-piperidinyl)-, (20(cis))-
66. Tylosin, 4a-o-de(2,6-dideoxy-3-c-methyl-alpha-l-ribo-hexopyranosyl)-20-deoxo-20-((3r,5s)-3,5-dimethyl-1-piperidinyl)-
| Molecular Weight | 869.1 g/mol |
|---|---|
| Molecular Formula | C46H80N2O13 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 12 |
| Exact Mass | 868.56604061 g/mol |
| Monoisotopic Mass | 868.56604061 g/mol |
| Topological Polar Surface Area | 186 Ų |
| Heavy Atom Count | 61 |
| Formal Charge | 0 |
| Complexity | 1420 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): Tilmicosin is available as an injectable formulation for the treatment of respiratory diseases in cattle and sheep and as a feed premix for the treatment and control of respiratory diseases in pigs.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
THERAP CAT (VET): Antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1683
MEDICATION (VET):The macrolides are used to treat both systemic and local infections. They are often regarded as alternatives to penicillins for the treatment of streptococcal and staphylococcal infections. General indications include upper respiratory tract infections, bronchopneumonia, bacterial enteritis, metritis, pyodermatitis, urinary tract infections, arthritis, and others. Formulations for treating mastitis are also available and often have the advantage of a short withholding time for milk. /Macrolides/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2091
MEDICATION (VET): Tilmicosin is a macrolide antibiotic synthesized from tylosin. It has an antibacterial spectrum similar to tylosin with enhanced activity against Pasteurella multocida and Pasteurella hemolitica. Tilmicosin is recommended for the treatment of bacterial pneumonia in young cattle ... .
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Tilmicosin, Summary Report (1) (January 1996). Available from, as of July 21, 2006: https://www.ema.europa.eu/ema/index.jsp?curl=pages/document_library/landing/document_library_search.jsp&murl=menus/document_library/document_library.jsp&mid
For more Therapeutic Uses (Complete) data for TILMICOSIN (8 total), please visit the HSDB record page.
/VET/: Tilmicosin is not labeled for use in female dairy cattle 20 months of age or older, veal calves, calves less than 1 month of age, or calves fed an all-milk diet.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Macrolides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
Toxicity and side effects are uncommon for most macrolides (except tilmicosin), although pain and swelling may develop at injection sites. Hypersensitivity reactions have occasionally been seen. ... Horses are sensitive to macrolide-induced GI disturbances that can be serious and even fatal. ... Tilmicosin is characterized by cardiac toxicity (tachycardia and decreased contractility). It is contraindicated in swine and should not be used in an extra-label manner. cattle hae died after IV injection of tilmicosin.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2091
Tilmicosin: All species: To avoid cardiotoxicity, tilmicosin should not be administered intravenously. Human: Injection of tilmicosin may be lethal. Although there is little information on the effects of tilmicosin in people, a variable susceptibility to cardiotoxic reactions in other species warrants caution with human exposure and close monitoring of the cardiovascular system, particularly after accidental injection. A physician should be consulted immediately in cases of accidental injection. Dogs: In laboratory dogs, tachycardia and decreased cardiac contractility have been noted in response to tilmicosin injection. Goats: Administration of tilmicosin to goats at intramuscular or subcutaneous doses >10 mg per kg of body weight (mg/kg) is likely to lead to toxicity. Horses: Administration of tilmicosin to horses at intramuscular or subcutaneous doses >10 mg/kg is likely to lead to toxicity. Pigs: Injection of tilmicosin into swine can be fatal as a result of cardiovascular toxicity. Administration of epinephrine to treat cardiovascular toxicity due to intravenous tilmicosin administration has been associated with an increased risk of death.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Macrolides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
/VET:/ In cattle, tilmicosin is distributed into milk at effective antibacterial concentrations for susceptible pathogens, but detectable concentrations in milk are maintained for many weeks (up to 42 days). Tilmicosin should not be administered to lactating dairy cattle because of impractical withdrawal times.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Macrolides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Pigs were given a dose of 154 or 400 mg (14)C-tilmicosin in the diet following a similar dose given for 5 days. The recovery of radioactivity was 4 to 6% in urine and 62 to 75% in feces. Radioactivity was detected in the bile but was not quantified.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
Pigs were administered a dose of 110 mg (14)C-tilmicosin in the diet over the course of one day. The recovery of radioactivity was 15% in the urine and 80% in the feces.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
Tilmicosin is administered SC. Absorption after injection is rapid ... .
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2090
Beulah cross lambs were administered a single subcutaneous dose of 20 mg/kg bw per day (14)C-tilmicosin. The major radioactive component in the liver, kidneys and urine was the parent drug, together with lesser amounts of T1 and T2, and minor amounts of other unidentified substances.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
For more Absorption, Distribution and Excretion (Complete) data for TILMICOSIN (13 total), please visit the HSDB record page.
Beulah cross lambs were administered a single subcutaneous dose of 20 mg/kg bw per day (14)C-tilmicosin. The major radioactive component in the liver, kidneys and urine was the parent drug, together with lesser amounts of T1 and T2, and minor amounts of other unidentified substances.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
Fischer-344 rats (10 males and 10 females) were given gavage doses of 50 mg/kg bw per day (14)C-tilmicosin for 5 days. An analysis of fecal radioactivity for the presence of the sulfate metabolite that was found in the feces of pigs revealed the presence of a similar compound, but quantification was not undertaken.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
Tilmicosin, labelled with (14)C in both the desmycosin macrolide ring and the piperidine ring, was given orally to 15 male and 15 female Fischer-344 rats. The dosage was 20 mg/kg bw per day for 3 days. In the liver, radiolabel corresponded to tilmicosin and a desmethyl derivative, T1 (demethylated in the mycaminose ring). The single radioactive substance identified in urine was unchanged tilmicosin, while in feces the major peak was parent compound with lesser amounts of desmethyl tilmicosin and a high molecular weight compound known to be present as an impurity in the administered substance, T2 (consisting of two macrolide rings and one piperidine ring).
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
In a summary of results obtained in cattle injected with (14)C-tilmicosin, it was reported that the radioactivity profile in the liver of treated rats was similar to that in the feces. In animals treated with a highly purified sample of tilmicosin, metabolite T2 was not detected in the liver, suggesting that its presence was a result of direct administration as a component of the drug substance. Radioactivity in the kidneys was essentially in the form of unchanged tilmicosin.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 38: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Tilmicosin (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v38je01.htm
For more Metabolism/Metabolites (Complete) data for TILMICOSIN (6 total), please visit the HSDB record page.
The plasma half-lives of macrolides usually are 1-3 hr, ... /Macrolides/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2091
Tilmicosin has in vitro activity against gram-positive organisms and mycoplasma and is active against certain gram-negative organisms, such as Hemophilus somnus, Mannheimia (Pasteurella) hemolytica, and Pasteurella multocida. However, M. hemolytica is more sensitive than P. multocida to tilmicosin. Other gram-negative organisms tested, including Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella, and Serratia species, are very resistant to tilmicosin. Some strains of Actinomyces also are extremely resistant to tilmicosin.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Macrolides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
The antimicrobial mechanism seems to be the same for all of the macrolides. They interfere with protein synthesis by reversibly binding to the 50 S subunit of the ribosome. They appear to bind at the donor site, thus preventing the translocation necessary to keep the peptide chain growing. The effect is essentially confined to rapidly dividing bacteria and mycoplasmas. Macrolides are regarded as being bacteriostatic, ... . Macrolides are significantly more active at higher pH ranges (7.8-8). /Macrolides/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2090
Macrolides have been reported to modify the host immune and inflammatory responses both in vivo and in vitro. /The authors/ examined the in vitro effect of the macrolides tilmicosin and tylosin, which are only used in the veterinary clinic, on the production of nitric oxide (NO), prostaglandin E2 (PGE2) and cytokines by lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and mouse peripheral blood mononuclear cells (PBMCs). Compared with 5 ug/mL, tilmicosin and tylosin concentrations of 10 ug/mL and 20 ug/mL significantly decreased the production of 6-keto-prostaglandin F1alpha (6-keto-PGF1alpha), PGE2, NO, tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-1beta and IL-6, and increased IL-10 production. Cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) gene expression were also significantly reduced. These results support the opinion that macrolides may exert an anti-inflammatory effect through modulating the synthesis of several mediators and cytokines involved in the inflammatory process.
PMID:16647840 Xing-Yuan Cao et al; International Journal of Antimicrobial Agents 27 (5): 431-8 (2006)