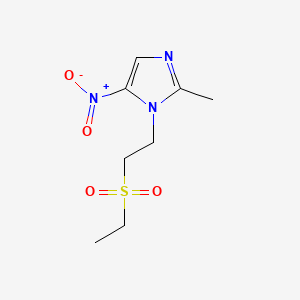

1. Bioshik
2. Cp 12,574
3. Cp 12574
4. Cp-12,574
5. Cp-12574
6. Cp12,574
7. Cp12574
8. Fasigin
9. Fasigyn
10. Fasigyne
11. Fasygin
12. Simplotan
13. Tricolam
1. 19387-91-8
2. Tindamax
3. Trimonase
4. Fasigyn
5. Tricolam
6. Fasigin
7. 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-1h-imidazole
8. Simplotan
9. 1-(2-ethylsulfonylethyl)-2-methyl-5-nitroimidazole
10. 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-1h-imidazole
11. Timidazole
12. Haisigyn
13. Cp-12574
14. 1h-imidazole, 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-
15. Cp-12,574
16. 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitroimidazole
17. 1-[2-(ethanesulfonyl)ethyl]-2-methyl-5-nitro-1h-imidazole
18. Tinidazol
19. Chebi:63627
20. 1h-imidazole, 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-
21. Nsc-758189
22. Mls000069717
23. 148159-84-6
24. Bioshik
25. 033kf7v46h
26. 1-(2-ethylsulfonylethyl)-2-methyl-5-nitro-imidazole
27. Ncgc00016741-01
28. Tinidazolum
29. Smr000058194
30. Sorquetan
31. Pletil
32. Tinidazole 100 Microg/ml In Acetonitrile
33. Cas-19387-91-8
34. Dsstox_cid_3676
35. Dsstox_rid_77141
36. Dsstox_gsid_23676
37. 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole
38. Glongyn
39. Amtiba
40. Cp 12574
41. Tinidazol [inn-spanish]
42. Tinidazolum [inn-latin]
43. 1h-imidazole, 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitro-, Radical Ion(1-)
44. Ccris 7621
45. Hsdb 7362
46. Sr-01000000210
47. Einecs 243-014-4
48. Brn 0618182
49. Symplotan
50. Ethyl (2-(2-methyl-5-nitro-1-imidazolyl)ethyl)sulfone
51. Unii-033kf7v46h
52. Fasigyntrade Mark
53. Tindamaxtrade Mark
54. Tinidazole,(s)
55. Simplotantrade Mark
56. Haisigyn (tn)
57. Tindamax (tn)
58. Prestwick_136
59. Cp12574
60. Tinidazole [usan:usp:inn:ban:jan]
61. Spectrum_001266
62. Opera_id_779
63. Specplus_000708
64. Tinidazole [mi]
65. Tinidazole [inn]
66. Tinidazole [jan]
67. Prestwick0_000766
68. Prestwick1_000766
69. Prestwick2_000766
70. Prestwick3_000766
71. Spectrum2_000648
72. Spectrum3_001512
73. Spectrum4_000230
74. Spectrum5_001715
75. Tinidazole [hsdb]
76. Tinidazole [usan]
77. Tinidazole [vandf]
78. Tinidazole [mart.]
79. Chembl1220
80. Tinidazole [usp-rs]
81. Tinidazole [who-dd]
82. Oprea1_342844
83. Schembl21141
84. Bspbio_000812
85. Bspbio_003183
86. Kbiogr_000899
87. Kbioss_001746
88. Methyl-5-nitro-1h-imidazole
89. 5-23-05-00067 (beilstein Handbook Reference)
90. Mls001146883
91. Mls001424192
92. Divk1c_006804
93. Spectrum1502127
94. Spbio_000655
95. Spbio_002751
96. Bpbio1_000894
97. Tinidazole (jp17/usp/inn)
98. 1-(ethylsulfonyl)-2-(2-methyl-5-nitroimidazolyl)ethane
99. Dtxsid4023676
100. Tinidazole [orange Book]
101. Kbio1_001748
102. Kbio2_001746
103. Kbio2_004314
104. Kbio2_006882
105. Kbio3_002683
106. Imidazole, 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-
107. Tinidazole [ep Monograph]
108. 1-(2-(ethylsulfonyl)ethyl)-2-
109. Hms1570i14
110. Hms1921l06
111. Hms2052c09
112. Hms2090b14
113. Hms2092d14
114. Hms2097i14
115. Hms2231e23
116. Hms3374p07
117. Hms3394c09
118. Hms3652a08
119. Hms3714i14
120. Pharmakon1600-01502127
121. Tinidazole [usp Monograph]
122. Zinc113446
123. Bcp20694
124. Hy-b0177
125. Tox21 110586
126. Tox21_110586
127. Bbl010767
128. Bdbm50248360
129. Ccg-39907
130. Mfcd00057217
131. Nsc758189
132. S4068
133. Stk801761
134. Akos000747070
135. Tinidazole 100 Microg/ml In Methanol
136. Tox21_110586_1
137. Ac-1618
138. Cs-2055
139. Db00911
140. Ks-5180
141. Mcule 2580495783
142. Nc00346
143. Nsc 758189
144. Ncgc00016741-02
145. Ncgc00016741-03
146. Ncgc00016741-04
147. Ncgc00016741-05
148. Ncgc00016741-06
149. Ncgc00016741-07
150. Ncgc00016741-09
151. Ncgc00022212-03
152. Ncgc00022212-04
153. Ncgc00022212-05
154. Sbi-0052674.p002
155. Ab00053176
156. Ft-0675241
157. Sw196447-4
158. T3058
159. Vu0239922-6
160. D01426
161. Tinidazole, Vetranal(tm), Analytical Standard
162. Ab00053176-16
163. Ab00053176_17
164. Ab00053176_18
165. A813669
166. Q1321320
167. Sr-01000000210-3
168. Sr-01000000210-5
169. Sr-01000000210-6
170. 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazol
171. Brd-k89125793-001-05-4
172. Z56763819
173. 1-(2'-(ethylsulfonyl)-ethyl]-2-methyl-5-nitroimidazole
174. Tinidazole, European Pharmacopoeia (ep) Reference Standard
175. Tinidazole, United States Pharmacopeia (usp) Reference Standard
176. Imidazole, 1-(2-(ethylsulfonyl)ethyl)-2-methyl-4-nitro-
177. Timtec-bb Sbb006917 Tinidazole 1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-1h-imidazol
| Molecular Weight | 247.27 g/mol |
|---|---|
| Molecular Formula | C8H13N3O4S |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 247.06267708 g/mol |
| Monoisotopic Mass | 247.06267708 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 345 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Tindamax |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tindamax pink oral tablets contain... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Mission Pharma |
| 2 of 4 | |
|---|---|
| Drug Name | Tinidazole |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tinidazole tablets contain 250 mg o... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Unique Pharm Labs; Novel Labs; Roxane |
| 3 of 4 | |
|---|---|
| Drug Name | Tindamax |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tindamax pink oral tablets contain... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Mission Pharma |
| 4 of 4 | |
|---|---|
| Drug Name | Tinidazole |
| PubMed Health | Tinidazole (By mouth) |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antibiotic, Antiprotozoal |
| Drug Label | Tinidazole is a synthetic antiprotozoal and antibacterial agent. It is 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, a second-generation 2-methyl-5-nitroimidazole, which has the following chemical structure:Tinidazole tablets contain 250 mg o... |
| Active Ingredient | Tinidazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Unique Pharm Labs; Novel Labs; Roxane |
Antitrichomonal Agents
National Library of Medicine, SIS; ChemIDplus Record for Tinidazole (19387-91-8), MESH heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION: Antiprotozoal (Trichomonas, Giardia); antiamebic; antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1685
Oral tinidazole is indicated in the treatment of amebic liver abscess caused by Entamoeba histolytica in both adults and pediatric patients older than three years of age. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Oral tinidazole is indicated in the treatment of intestinal amebiasis caused by Entamoeba histolytica in both adults and pediatric patients older than three years of age. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
For more Therapeutic Uses (Complete) data for TINIDAZOLE (6 total), please visit the HSDB record page.
Tinidazole is distributed into breast milk at concentrations similar to those in serum. Because tinidazole can be detected in breast milk for up to 72 hours after administration, interruption of breast-feeding during tinidazole therapy and for three days following the last dose is recommended.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2832
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2832
Tinidazole crosses the placenta, and enters the fetal circulation. Therefore, it should not be administered to pregnant women during the first trimester.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2832
Adverse effects occurring in 1% or more of patients receiving tinidazole include GI effects (metallic/bitter taste, nausea, anorexia, dyspepsia/cramps/epigastric discomfort, vomiting, constipation) and nervous system effects (weakness/fatigue/malaise, dizziness, headache).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 875
For more Drug Warnings (Complete) data for TINIDAZOLE (11 total), please visit the HSDB record page.
For the treatment of trichomoniasis caused by T. vaginalis in both female and male patients. Also for the treatment of giardiasis caused by G. duodenalis in both adults and pediatric patients older than three years of age and for the treatment of intestinal amebiasis and amebic liver abscess caused by E. histolytica in both adults and pediatric patients older than three years of age.
FDA Label
Tinidazole is a synthetic antiprotozoal agent. Tinidazole demonstrates activity both in vitro and in clinical infections against the following protozoa: Trichomonas vaginalis, Giardia duodenalis (also termed G. lamblia), and Entamoeba histolytica. Tinidazole does not appear to have activity against most strains of vaginal lactobacilli.
Antitrichomonal Agents
Agents used to treat trichomonas infections. (See all compounds classified as Antitrichomonal Agents.)
Alkylating Agents
Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning. Many are used as antineoplastic agents, but most are very toxic, with carcinogenic, mutagenic, teratogenic, and immunosuppressant actions. They have also been used as components in poison gases. (See all compounds classified as Alkylating Agents.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XD02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XD - Imidazole derivatives
J01XD02 - Tinidazole
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AB - Nitroimidazole derivatives
P01AB02 - Tinidazole
Absorption
Rapidly and completely absorbed under fasting conditions. Administration with food results in a delay in Tmax of approximately 2 hours and a decline in Cmax of approximately 10% and an AUC of 901.6 ± 126.5 mcg hr/mL.
Route of Elimination
Tinidazole crosses the placental barrier and is secreted in breast milk. Tinidazole is excreted by the liver and the kidneys. Tinidazole is excreted in the urine mainly as unchanged drug (approximately 20-25% of the administered dose). Approximately 12% of the drug is excreted in the feces.
Volume of Distribution
50 L
Tinidazole is distributed into virtually all tissues and body fluids. Tinidazole also crosses the blood-brain barrier, placental barrier and is distributed into breast milk. Volume of distribution (VolD): about 50 L.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Under fasted conditions tinidazole is rapidly and completely absorbed. Administration with food resulted in a delay in Tmax of approximately 2 hours and a decline in Cmax of approximately 10% and an AUC of 901.6 + or - 126.5 ug hr/mL.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Time to peak concentration: 1.6 (+ or - 0.7 hours)
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Elimination: Renal: 20 to 25% as unchanged drug. Fecal: 12%. In hemodialysis: 43% eliminated during 6 hour hemodialysis session.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
For more Absorption, Distribution and Excretion (Complete) data for TINIDAZOLE (6 total), please visit the HSDB record page.
Hepatic, mainly via CYP3A4. Tinidazole, like metronidazole, is significantly metabolized in humans prior to excretion. Tinidazole is partly metabolized by oxidation, hydroxylation and conjugation. Tinidazole is the major drug-related constituent in plasma after human treatment, along with a small amount of the 2-hydroxymethyl metabolite.
Tinidazole, like metronidazole, is significantly metabolized in humans prior to excretion. Tinidazole is partly metabolized by oxidation, hydroxylation and conjugation. Tinidazole is the major drug-related constituent in plasma after human treatment, along with a small amount of the 2-hydroxymethyl metabolite. Tinidazole is biotransformed mainly by CYP3A4. In an in vitro metabolic drug interaction study, tinidazole concentrations of up to 75 ug/mL did not inhibit the enzyme activities of CYP1A2, CYP2B6, CYP2C9, CYP2D6, CYP2E1 and CYP3A4.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2670
The elimination half-life is 13.21.4 hours and the plasma half-life is 12 to 14 hours.
Elimination: 13.2 (+ or - 1.4) hours. Plasma: approximately 12 to 14 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831
Tinidazole is a prodrug and antiprotozoal agent. The nitro group of tinidazole is reduced in Trichomonas by a ferredoxin-mediated electron transport system. The free nitro radical generated as a result of this reduction is believed to be responsible for the antiprotozoal activity. It is suggested that the toxic free radicals covalently bind to DNA, causing DNA damage and leading to cell death. The mechanism by which tinidazole exhibits activity against Giardia and Entamoeba species is not known, though it is probably similar.
The nitro group of tinidazole is reduced by cell extracts of Trichomonas. As a result of this reduction a free nitro radical is generated which may be responsible for the antiprotozoal activity. The mechanism by which tinidazole exhibits activity against Giardia and Entamoeba species is not known.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2831