

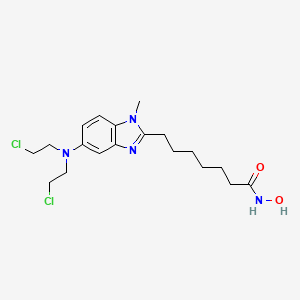

1. Edo-s101
1. Edo-s101
2. 1236199-60-2
3. Minomustine
4. Edo-s 101
5. Edo-s-101
6. Tinostamustine [usan]
7. Tinostamustine(edo-s101)
8. 29dki2h2ny
9. 7-(5-(bis(2-chloroethyl)amino)-1-methyl-1h-benzo[d]imidazol-2-yl)-n-hydroxyheptanamide
10. 7-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]-n-hydroxyheptanamide
11. 1h-benzimidazole-2-heptanamide, 5-[bis(2-chloroethyl)amino]-n-hydroxy-1-methyl-
12. 1h-benzimidazole-2-heptanamide, 5-(bis(2-chloroethyl)amino)-n-hydroxy-1-methyl-
13. Starbld0018955
14. Unii-29dki2h2ny
15. Tinostamustine (usan/inn)
16. Tinostamustine [inn]
17. Schembl7915449
18. Tinostamustine [who-dd]
19. Chembl3989941
20. Bcp20331
21. Ex-a1322
22. Bdbm50569838
23. Zinc68244536
24. Cs-6484
25. Db15147
26. Sb19172
27. Be170657
28. Hy-101780
29. S8769
30. D11182
31. E76854
32. 7-(5-(bis(2-chloroethyl)amino)-1-methyl-1h-benzimidazol-2-yl)-n-hydroxyheptanamide
| Molecular Weight | 415.4 g/mol |
|---|---|
| Molecular Formula | C19H28Cl2N4O2 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 12 |
| Exact Mass | 414.1589315 g/mol |
| Monoisotopic Mass | 414.1589315 g/mol |
| Topological Polar Surface Area | 70.4 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 438 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |