


1. Gyno-trosyd
2. Monistat 1-day
3. Mykontral
4. Trosderm
5. Trosid
6. Trosyd
7. Trosyl
8. Uk 20,349
9. Vagistat
10. Vagistat-1
1. 65899-73-2
2. Vagistat
3. Vagistat-1
4. Trosyd
5. Trosyl
6. Gyno-trosyd
7. Fungibacid
8. Zoniden
9. Tioconazol [inn-spanish]
10. Tioconazolum [inn-latin]
11. Uk-20349
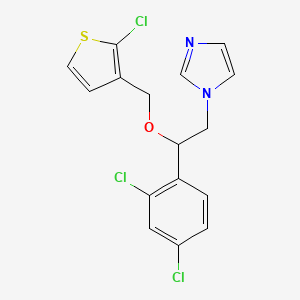
12. 1-(2-((2-chlorothiophen-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-1h-imidazole
13. Uk-20,349
14. Tioconazolum
15. Nsc-759169
16. 1-[2-[(2-chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole
17. Tioconazol
18. Chebi:77898
19. Mfcd00057276
20. S57y5x1117
21. Ncgc00167430-01
22. Tz-3
23. 1-{2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole
24. 1-{2-[(2-chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1h-imidazole
25. 1h-imidazole, 1-(2-((2-chloro-3-thienyl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-
26. Dsstox_cid_26619
27. Dsstox_rid_81771
28. Dsstox_gsid_46619
29. New Straitus
30. 1-[2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole
31. Smr001550005
32. (+-)-tioconazole
33. Cas-65899-73-2
34. Vagistat-1 (tn)
35. Uk 20349
36. Sr-05000001939
37. Einecs 265-973-8
38. Brn 0573867
39. Tioconazole (jan/usp/inn)
40. Unii-s57y5x1117
41. 1h-imidazole, 1-[2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-
42. 1-[(2s)-2-[(2-chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole
43. 1-(2,4-dichloro-beta-((2-chloro-3-thenyl)-oxy)phenethyl)imidazole
44. (+-)-1-(2,4-dichloro-beta-((2-chloro-3-ethenyl)oxy)phenethyl)imidazole
45. Tioconazole, 97%
46. Tioconazole [usan:usp:inn:ban:jan]
47. Tioconazole [mi]
48. 1-(2,4-dichloro-(beta-(2-chloro-3-thenyl)oxy)phenethyl)imidazole
49. Tioconazole [inn]
50. Tioconazole [jan]
51. (.+/-.)-tioconazole
52. Tioconazole [inci]
53. Tioconazole [usan]
54. Tioconazole [vandf]
55. Tioconazole [mart.]
56. Schembl41354
57. Tioconazole [usp-rs]
58. Tioconazole [who-dd]
59. 5-23-04-00324 (beilstein Handbook Reference)
60. Mls003899209
61. Mls004712040
62. Mls006010907
63. Chembl1200438
64. Dtxsid3046619
65. Tioconazole [orange Book]
66. Tioconazole For System Suitability
67. Hms2090b12
68. Hms2093p11
69. Hms3655k22
70. Hms3712i10
71. Pharmakon1600-01505581
72. Tioconazole [ep Monograph]
73. Tioconazole [usp Impurity]
74. Tioconazole [usp Monograph]
75. Amy32535
76. Hy-b0319
77. Tox21_112434
78. Bdbm50370218
79. Dl-515
80. Nsc759169
81. S1910
82. Akos015906500
83. Tox21_112434_1
84. Ccg-213487
85. Db01007
86. Ks-5114
87. Nsc 759169
88. (+-)-1-[2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1h-imidazole
89. Ncgc00167430-02
90. Ncgc00167430-03
91. Ncgc00167430-04
92. Ac-13425
93. Ac-31721
94. Sbi-0206824.p001
95. Ft-0630756
96. Sw198590-2
97. C08082
98. D00890
99. D83125
100. Ab01275523-01
101. Ab01275523_02
102. Ab01275523_03
103. Tioconazole, Vetranal(tm), Analytical Standard
104. 899t732
105. A835270
106. Q260326
107. Sr-05000001939-1
108. Sr-05000001939-2
109. Brd-a33084557-001-01-7
110. Tioconazole, United States Pharmacopeia (usp) Reference Standard
111. 1-[2,4-dichloro-.beta.-[(2-chloro-3-thenyl)-oxy]phenethyl]imidazole
112. (.+/-.)-1-(2,4-dichloro-.beta.-((2-chloro-3-ethenyl)oxy)phenethyl)imidazole
113. 1-[2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1h-imidazole #
114. 1-{2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1h-imidazole
115. Tioconazole For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 387.7 g/mol |
|---|---|
| Molecular Formula | C16H13Cl3N2OS |
| XLogP3 | 5.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 385.981417 g/mol |
| Monoisotopic Mass | 385.981417 g/mol |
| Topological Polar Surface Area | 55.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 379 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Tioconazole |
| PubMed Health | Tioconazole (Topical route) |
| Drug Classes | Imidazole |
| Active Ingredient | Tioconazole |
| Dosage Form | Ointment |
| Route | Vaginal |
| Strength | 6.5% |
| Market Status | Over the Counter |
| Company | Perrigo |
| 2 of 4 | |
|---|---|
| Drug Name | Vagistat-1 |
| Active Ingredient | Tioconazole |
| Dosage Form | Ointment |
| Route | Vaginal |
| Strength | 6.5% |
| Market Status | Over the Counter |
| Company | Novartis |
| 3 of 4 | |
|---|---|
| Drug Name | Tioconazole |
| PubMed Health | Tioconazole (Topical route) |
| Drug Classes | Imidazole |
| Active Ingredient | Tioconazole |
| Dosage Form | Ointment |
| Route | Vaginal |
| Strength | 6.5% |
| Market Status | Over the Counter |
| Company | Perrigo |
| 4 of 4 | |
|---|---|
| Drug Name | Vagistat-1 |
| Active Ingredient | Tioconazole |
| Dosage Form | Ointment |
| Route | Vaginal |
| Strength | 6.5% |
| Market Status | Over the Counter |
| Company | Novartis |
For the local treatment of vulvovaginal candidiasis (moniliasis).
FDA Label
Tioconazole is a broad-spectrum imidazole antifungal agent that inhibits the growth of human pathogenic yeasts. Tioconazole exhibits fungicidal activity in vitro against Candida albicans, other species of the genus Candida, and against Torulopsis glabrata. Tioconazole prevents the growth and function of some fungal organisms by interfering with the production of substances needed to preserve the cell membrane. This drug is effective only for infections caused by fungal organisms. It will not work for bacterial or viral infections.
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AC - Imidazole and triazole derivatives
D01AC07 - Tioconazole
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AF - Imidazole derivatives
G01AF08 - Tioconazole
Absorption
Systemic absorption following a single intravaginal application of tioconazole in nonpregnant patients is negligible.
Orally administered tioconazole is extensively metabolized. The major metabolites are glucuronide conjugates.
Tioconazole interacts with 14-α demethylase, a cytochrome P-450 enzyme that converts lanosterol to ergosterol, an essential component of the yeast membrane. In this way, tioconazole inhibits ergosterol synthesis, resulting in increased cellular permeability. Tioconazole may also inhibit endogenous respiration, interact with membrane phospholipids, inhibit the transformation of yeasts to mycelial forms and the uptake of purine, impair triglyceride and/or phospholipid biosynthesis, and inhibit the movement of calcium and potassium ions across the cell membrane by blocking the ion transport pathway known as the Gardos channel.