
1. Navane
2. Thiothixene
3. Tiotixene
1. Trans-thiothixene
2. Thiothixene
3. (e)-thiothixene
4. 3313-27-7
5. Orbinamon
6. 5591-45-7
7. Thiothixene, (e)-
8. P-4657a
9. Navan
10. 7ue42hf37r
11. Cp-12,252-1 Base
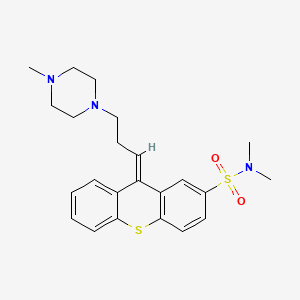
12. (9e)-n,n-dimethyl-9-[3-(4-methylpiperazin-1-yl)propylidene]thioxanthene-2-sulfonamide
13. 9h-thioxanthene-2-sulfonamide,n,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]-
14. P 4657b
15. P-4657 B
16. P-4657-b
17. N,n-dimethyl-9-(3-(4-methylpiperazin-1-yl)propylidene)-9h-thioxanthene-2-sulfonamide
18. Smr000653538
19. Unii-7ue42hf37r
20. Trans-tiotixen
21. Nsc108165
22. 9h-thioxanthene-2-sulfonamide, N,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]-
23. N,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]thioxanthene-2-sulfonamide
24. Ncgc00015998-04
25. Spectrum5_001150
26. Lopac-t-0780
27. Bspbio_002022
28. Mls001146956
29. Mls001360493
30. Schembl718331
31. Spectrum1500576
32. 2-(dimethylsulfamoyl)-[9-(4-methyl-1-piperazinyl)propylidene]thioxanthene
33. Chembl1336727
34. Bdbm86187
35. Chebi:93708
36. Hms501a17
37. (e)-n,n-dimethyl-9-(3-(4-methylpiperazin-1-yl)propylidene)-9h-thioxanthene-2-sulfonamide
38. N,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]thiaxanthene-2-sulfonamide
39. Dtxsid401316424
40. Hms1921e13
41. Hms2092m15
42. Hms2230h09
43. Pharmakon1600-01500576
44. (e)-thiothixene [usp-rs]
45. Nsc_5454
46. Ccg-39271
47. Nsc757350
48. Pdsp1_000169
49. Pdsp1_000550
50. Pdsp2_000168
51. Pdsp2_000548
52. Thiothixene Trans-ismer [mi]
53. Zinc29747244
54. Akos024283092
55. At12061
56. Nsc-757350
57. Idi1_000335
58. Thioxanthene-2-sulfonamide, N,n-dimethyl-9-(3-(4-methyl-1-piperazinyl)propylidene)-, (e)-
59. Trans-n,n-dimethyl-9-(3-(4-methyl-piperazinyl)propylidene)thioxanthene-2-sulfonamide
60. Ncgc00015998-01
61. Ncgc00015998-02
62. Ncgc00015998-03
63. Ncgc00015998-06
64. Ncgc00094793-01
65. Ncgc00094793-02
66. Ncgc00094793-04
67. Sbi-0051534.p003
68. Cas_22189-31-7
69. Ab00052109_11
70. L001204
71. Sr-05000002078
72. Sr-05000002078-1
73. Brd-k97309399-001-02-9
74. Brd-k97309399-001-05-2
75. Q27268863
76. Wln: T C666 Bs Iyj Fswn1&1 Iu3- At6n Dntj D1
77. (9e)-n,n-dimethyl-9-[3-(4-methylpiperazin-1-yl)propylidene]-9h-thioxanthene-2-sulfonamide
78. 9h-thioxanthene-2-sulfonamide,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]-
79. 9h-thioxanthene-2-sulfonamide,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]-, (z)-
80. Thioxanthene-2-sulfonamide,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]-
81. 9h-thioxanthene-2-sulfonamide, N,n-dimethyl-9-(3-(4-methyl-1-piperazinyl)propylidene)-, (9e)-
82. Cis-thiothixene N,n-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]thioxanthene-2-sulfonamide
83. N,n-dimethyl-9-(3-(4-methyl-1-piperazinyl)propylidene)-9h-thioxanthene-2-sulfonamide, (e)-
| Molecular Weight | 443.6 g/mol |
|---|---|
| Molecular Formula | C23H29N3O2S2 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 443.17011952 g/mol |
| Monoisotopic Mass | 443.17011952 g/mol |
| Topological Polar Surface Area | 77.5 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 711 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Thiothixene |
| PubMed Health | Thiothixene (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | Thiothixene is a thioxanthene derivative. Specifically, it is the cis isomer of N,N-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]thioxanthene-2-sulfonamide. It may be represented by the following structural formula:The thioxanthenes differ from... |
| Active Ingredient | Thiothixene |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 1mg; 5mg; 2mg; 10mg |
| Market Status | Prescription |
| Company | Sandoz; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Thiothixene |
| PubMed Health | Thiothixene (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | Thiothixene is a thioxanthene derivative. Specifically, it is the cis isomer of N,N-dimethyl-9-[3-(4-methyl-1-piperazinyl)propylidene]thioxanthene-2-sulfonamide. It may be represented by the following structural formula:The thioxanthenes differ from... |
| Active Ingredient | Thiothixene |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 1mg; 5mg; 2mg; 10mg |
| Market Status | Prescription |
| Company | Sandoz; Mylan |
Antipsychotic Agents; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings. Citalopram. Online file (MeSH, 2018). Available from, as of February 2, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Thiothixene is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 2, 2018: https://clinicaltrials.gov/
Thiothixene capsules are effective in the management of schizophrenia. /Included in US product label/
NIH; DailyMed. Current Medication Information for Thiothixene Capsule (Updated: March 24, 2017). Available from, as of February 7, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=265f456a-b7eb-41ba-8eb4-fa417a5191cb
Experience with the drug in treating neurotic conditions is limited and does not indicate that thiothixene is likely to have advantages over anxiolytic agents, butyrophenones, phenothiazines, or chlorprothixene (no longer commercially available in the US). Thiothixene has not been evaluated in the management of behavioral complications in mentally retarded patients.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
/BOXED WARNING/ Increased Mortality in Elderly Patients with Dementia-Related Psychosis: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thiothixene is not approved for the treatment of patients with dementia-related psychosis.
NIH; DailyMed. Current Medication Information for Thiothixene Capsule (Updated: March 24, 2017). Available from, as of February 7, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=265f456a-b7eb-41ba-8eb4-fa417a5191cb
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs, including thiothixene. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
NIH; DailyMed. Current Medication Information for Thiothixene Capsule (Updated: March 24, 2017). Available from, as of February 7, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=265f456a-b7eb-41ba-8eb4-fa417a5191cb
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs, including thiothixene. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
NIH; DailyMed. Current Medication Information for Thiothixene Capsule (Updated: March 24, 2017). Available from, as of February 7, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=265f456a-b7eb-41ba-8eb4-fa417a5191cb
The most frequent adverse effects of thiothixene are drowsiness (which usually is mild and subsides with continuation of therapy) and extrapyramidal symptoms. Like propylpiperazine phenothiazines, thiothixene is more likely to produce akathisia and dystonia than parkinson-like syndromes. Generally, extrapyramidal effects can be controlled by reducing the dosage of thiothixene and/or administering an antiparkinsonian drug.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
For more Drug Warnings (Complete) data for Thiothixene (21 total), please visit the HSDB record page.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Fifty-nine plasma thiothixene concentrations were measured in 42 patients as part of routine therapeutic drug monitoring. Data collection included concomitant medications, smoking history, and demographic variables. A retrospective analysis was performed to assess the effect of these parameters on oral thiothixene clearance. When groups of patients were categorized by concomitant medications (i.e., no interacting drugs, enzyme/clearance inducers, and enzyme/clearance inhibitors), thiothixene clearance was found to be significantly increased by enzyme inducing drugs (e.g., anticonvulsants) and decreased by clearance inhibiting agents (e.g., cimetidine). Tobacco smoking significantly increased the hepatic clearance of thiothixene within the no interactions and inhibitor groups, but not in the inducer group. Significantly more patients in the inducer group had nondetectable plasma concentrations of thiothixene than the other groups. When the entire patient population was dichotomized by age, patients less than 50 years old had a significantly greater mean clearance (48.2 +/- 37.8 liters/min) versus those greater than or equal to 50 (20.0 +/- 12.6 liters/min). Men in this cohort exhibited a significantly higher clearance (49.2 +/- 38.7 liters/min) than did the women (22.0 +/- 13.5 liters/min). By taking into account these potential sources of pharmacokinetic variability when monitoring plasma thiothixene concentrations, more appropriate dosing of thiothixene may be achieved. Controlled, prospective studies are needed to validate these findings.
PMID:1765572 Ereshefsky et al; J Clin Psychopharmacol 11 (5): 296-301 (1991)
Thiothixene is widely distributed into body tissues and may remain in the body for several weeks following administration.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
Thiothixene is well absorbed from the GI tract. Therapeutic response may occur within a few days to several weeks following oral administration of the drug. Plasma concentrations required for therapeutic effects are not known.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
Two experiments are reported in which acute single test dose levels of thiothixene (Navane) were correlated with age. In the first study 20 mg oral doses were given to 28 male subjects and serum levels were drawn 2 hr later. Mean age was 30 and correlation of serum level with age was 0.43, P less than 0.02. In a second older group with a mean age of 41, 10 mg oral doses were given to 25 subjects. A correlation with age of 0.41, P less than 0.05 was obtained with age. In prior work such acute levels have been found to correlate with steady-state serum levels and with clinical response to the medication. ...
PMID:6791222 Yesavage JA et al; Psychopharmacology (Berl) 74 (2): 170-2 (1981)
Thiothixene is metabolized in the liver and is excreted mainly in feces via biliary elimination as unchanged drug and as the demethyl, sulfoxide, demethylated sulfoxide, and hydroxylated thiothixene derivatives.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017