


1. Klisyri
2. Kx-01
3. Kx2-391
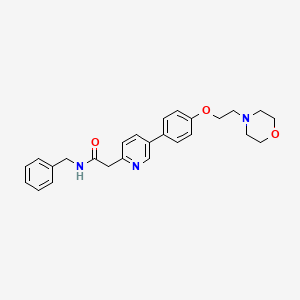
4. N-benzyl-2-(5-(4-(2-(4-morpholinyl)ethoxy)phenyl)-2-pyridinyl)acetamide
5. Tirbanibulin
1. Kx2-391
2. 897016-82-9
3. Tirbanibulin
4. Kx-01
5. Klisyri
6. Kx-2-391
7. Kx-2391
8. Kx01
9. Tirbanibulin [usan]
10. Kx2391
11. Chembl571546
12. 4v9848rs5g
13. Kx2-391;kx-01
14. 2-pyridineacetamide, 5-(4-(2-(4-morpholinyl)ethoxy)phenyl)-n-(phenylmethyl)-
15. N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide
16. 2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]-~{n}-(phenylmethyl)ethanamide
17. Unii-4v9848rs5g
18. N-benzyl-2-(5-(4-(2-morpholin-4-ylethoxy)phenyl)pyridin-2-yl)acetamide
19. Kx 01
20. Tirbanibulin (usan/inn)
21. Tirbanibulin [inn]
22. Kx2-391 (tirbanibulin)
23. Tirbanibulin (kx2-391)
24. Mls006011272
25. Schembl153779
26. Tirbanibulin [who-dd]
27. Gtpl7957
28. Dtxsid30237862
29. Hms3656j15
30. Hms3673e15
31. Tirbanibulin [orange Book]
32. Bcp02845
33. Ex-a2434
34. Xkb01682
35. Bdbm50303801
36. Nsc756643
37. Nsc800779
38. S2700
39. Who 10864
40. Zinc43152787
41. N-benzyl-2-(5-{4-[2-(morpholin-4-yl)ethoxy]phenyl}pyridin-2-yl)acetamide
42. Bcp9000828
43. Ccg-264983
44. Cs-0248
45. Db06137
46. Kx2-391; Kx-01
47. Nsc-756643
48. Nsc-800779
49. Sb16619
50. Ncgc00346644-01
51. Ncgc00346644-05
52. Ac-35458
53. As-73245
54. Hy-10340
55. Kx 2-391
56. Smr004703022
57. Db-119272
58. Sw219670-1
59. C77028
60. D11691
61. A915990
62. Brd-k29968218-001-01-6
63. Q27888424
64. 2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl)-n-benzylacetamide
65. 2-(5-(4-(2-morpholinoethoxyl)phenyl)pyridin-2-yl)-n-benzylacetamide
66. 2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]-n-(phenylmethyl)acetamide
67. 2-pyridineacetamide,5-[4-[2-(4-morpholinyl)ethoxy]phenyl]-n-(phenylmethyl)-
68. 5-[4-[2-(4-morpholinyl)ethoxy]phenyl]-n-(phenylmethyl)-2-pyridineacetamide
69. Dn0
| Molecular Weight | 431.5 g/mol |
|---|---|
| Molecular Formula | C26H29N3O3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 431.22089180 g/mol |
| Monoisotopic Mass | 431.22089180 g/mol |
| Topological Polar Surface Area | 63.7 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 540 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tirbanibulin is indicated for the topical treatment of actinic keratosis on the face or scalp.
Klisyri is indicated for the field treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis (Olsen grade 1) of the face or scalp in adults.
In clinical trials composed comprising patients with actinic keratosis of the face or scalp, tirbanibulin promoted complete clearance of actinic keratosis lesions at day 57 in treated areas in 44-54% of patients compared to 5-13% of patients who received the placebo. Actinic keratosis is a chronic, pre-malignant condition characterized by lesions and proliferation of neoplastic keratinocytes. Tirbanibulin mediates an anti-proliferative effect by inhibiting tubulin polymerization and Src kinase signalling. Tirbanibulin inhibited primary tumour growth and metastasis in many preclinical animal models of cancer. In human triple-negative breast cancer, or estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2)-negative tumour, xenografts, tirbanibulin suppressed tumour growth and metastasis. Tirbanibulin was also shown to restore functional ER expression in ER-negative breast tumours. Tirbanibulin promoted synergistic tumour growth inhibition of breast cancer cell lines when used in combination with tamoxifen and paclitaxel. In a clinical trial comprising patients with advanced solid tumours, dose-limiting toxicities of tirbanibulin included elevated liver transaminases, neutropenia and fatigue.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
D06BX03
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BX - Other chemotherapeutics
D06BX03 - Tirbanibulin
Absorption
Tirbanibulin demonstrates good oral bioavailability. Following topical administration of doses ranging from 54 to 295 mg on the face or scalp, the steady-state concentration of tirbanibulin was achieved by 72 hours. At five days following initial administration, the mean Cmax was 0.340.30 ng/mL in subjects who received topical treatment on the face and 0.180.10 ng/mL in subjects who received topical treatment on the scalp. The mean AUC24 was 5.03.9 h x ng/mL in subjects who received topical treatment on the face and 3.21.9 h x ng/mL in subjects who received topical treatment on the scalp. The median Tmax was about seven hours.
Route of Elimination
There is limited information on the route of elimination of tirbanibulin.
Volume of Distribution
There is limited information on the volume of distribution of tirbanibulin. In mouse HT29 xenograft studies, the tissue to plasma ration of tirbanibulin was 1.52.
Clearance
There is limited information on the clearance rate of tirbanibulin.
_In vitro_, tirbanibulin is mainly metabolized by CYP3A4, and to a lesser extent, CYP2C8. In adult subjects with actinic keratosis, detected metabolites were KX2-5036 and KX2-5163, which were pharmacologically inactive metabolites with the highest plasma concentrations of 0.09 ng/mL and 0.12 ng/mL, respectively.
The half-life is about 4 hours.
Src tyrosine kinases regulate normal cell growth: the expression of Src kinase is upregulated during the normal hair cycle during the proliferative anagen phase. Additionally, Src tyrosine kinases act as key modulators of cancer cell proliferation, survival, angiogenesis, migration, invasion and metastasis. Src is frequently upregulated in various epithelial tumours including colon, breast and pancreas compared with the adjacent normal tissues. The expression and activity of Src are also enhanced in human actinic keratosis, which is characterized by hyperproliferative premalignant skin lesions. The pathogenesis of actinic keratosis commonly involves skin inflammation, oxidative stress, immunosuppression, impaired apoptosis, mutagenesis, dysregulation of keratinocyte growth and proliferation, and tissue remodelling. _In vitro_ studies suggest that Src plays a predominant role in the early stages of human skin tumour development, rather than at later stages of tumour progression. The exact mechanism of tirbanibulin as a topical treatment of actinic keratosis has not been fully elucidated; however, it mainly works by inhibiting fast proliferating cells. Tirbanibulin is a non-ATP competitive Src kinase inhibitor and tubulin polymerization inhibitor. It binds to the peptide substrate binding site of Src, a primary target of tirbanibulin, and blocking its downstream signalling pathways that promote cancer cell migration, proliferation, and survival. Tublin is responsible for cell migration, protein transport, and mitosis: tibranibulin directly binds to the colchicine-binding site of beta-tubulin and causes induces tubulin depolymerization. It is also hypothesized that inhibition of Src can also contribute to the inhibitory effects on microtubule polymerization. At low nanomolar concentrations, tirbanibulin induces G2/M phase cell cycle arrest in a reversible and dose-dependent manner. By inhibiting microtubule polymerization, tirbanibulin also induces mitotic catastrophe.