


1. Agrastat
2. L 700,462
3. L 700462
4. L-700,462
5. L-700462
6. L700,462
7. Mk 383
8. Mk-383
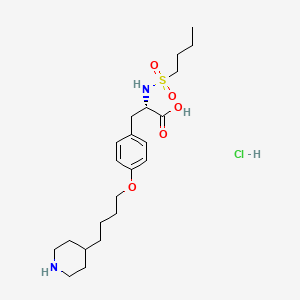
9. N-(butylsulfonyl)-o-(4-(4-piperidyl)butyl)-l-tyrosine
10. Tirofiban
11. Tirofiban Hydrochloride
12. Tirofiban Hydrochloride Monohydrate
1. Tirofiban Hydrochloride
2. 142373-60-2
3. Tirofiban Hcl
4. Tirofiban (hydrochloride)
5. Tirofiban Hydrochloride Anhydrous
6. Ike1p4x57j
7. (s)-2-(butylsulfonamido)-3-(4-(4-(piperidin-4-yl)butoxy)phenyl)propanoic Acid Hydrochloride
8. Mk 383
9. L 700462
10. 142373-60-2 (hcl)
11. L-tyrosine, N-(butylsulfonyl)-o-(4-(4-piperidinyl)butyl)-, Monohydrochloride
12. Tirofibanhydrochloridemonohydrate
13. N-(butylsulfonyl)-o-[4-(4-piperidinyl)butyl]-l-tyrosine Hydrochloride
14. L-700462
15. Mk-383
16. Unii-ike1p4x57j
17. N-(butylsulfonyl)-o-(4-(4-piperidinyl)butyl)-l-tyrosine Monohydrochloride
18. Chembl1704
19. Schembl41327
20. Dtxsid70931418
21. 2-(butylsulfonylamino)-3-[4-[4-(4-piperidinyl)butoxy]phenyl]propanoic Acid Hydrochloride
22. Amy23379
23. Ex-a2875
24. Hy-17369a
25. Mfcd00868210
26. Akos025311245
27. Tirofiban Hydrochloride [who-dd]
28. Cs-0009551
29. T3640
30. Tirofiban Hydrochloride Anhydrous [mi]
31. 915t405
32. Q27280770
33. (s)-2-(butylsulfonamido)-3-(4-(4-(piperidin-4-yl)butoxy)phenyl)propanoicacidhydrochloride
34. L-tyrosine, N-(butylsulfonyl)-o-(4-(4-piperidinyl)butyl)-, Hydrochloride (1:1)
35. N-(butane-1-sulfonyl)-o-[4-(piperidin-4-yl)butyl]tyrosine--hydrogen Chloride (1/1)
36. (2s)-2-(butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic Acid;hydrochloride
| Molecular Weight | 477.1 g/mol |
|---|---|
| Molecular Formula | C22H37ClN2O5S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 14 |
| Exact Mass | 476.2111712 g/mol |
| Monoisotopic Mass | 476.2111712 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)