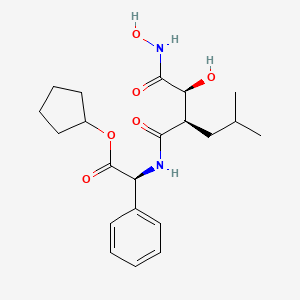

1. 2-(2-(hydroxy(hydroxycarbamoyl)methyl)-4-methylpentanoylamino)-2-phenylethanoic Acid Cyclopentyl Ester
2. Chr 2797
3. Chr-2797
4. Chr2797
1. 238750-77-1
2. Chr 2797
3. Chr-2797
4. Tosedostat (chr2797)
5. Kzk563j2uw
6. Unii-kzk563j2uw
7. (s)-cyclopentyl 2-((r)-2-((s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl)-4-methylpentanamido)-2-phenylacetate
8. Cyclopentyl (s)-2-((r)-2-((s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl)-4-methylpentanamido)-2-phenylacetate
9. Tosedostat [inn]
10. Tosedostat [usan:inn]
11. Bb-76163
12. Tosedostat (usan/inn)
13. Tosedostat [usan]
14. Chr-2797(tosedostat)
15. Chr-2797; Tosedostat
16. Chr2797 - Tosedostat
17. Tosedostat [who-dd]
18. Mls006011104
19. Chembl2103847
20. Schembl19236550
21. Chebi:95044
22. Dtxsid60178577
23. Bcpp000283
24. Hms3414a15
25. Hms3678a15
26. Cyclopentyl (2s)-2-[[(2r)-2-[(1s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoyl]amino]-2-phenylacetate
27. Ex-a2628
28. Bdbm50277143
29. Nsc806020
30. Zinc13914293
31. Akos024457707
32. At25061
33. Bcp9000524
34. Db11781
35. Nsc-806020
36. Ncgc00263175-01
37. Ac-35830
38. As-55983
39. Hy-14807
40. Smr004676647
41. Cs-0003570
42. D10026
43. 750t771
44. A857789
45. Brd-k92241597-001-01-1
46. Q27166812
47. Alpha-[[(2r)-2-[(1s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl]-4-methyl-1-oxopentyl]amino]benzeneacetic Acid Cyclopentyl Ester
48. Benzeneacetic Acid, .alpha.-(((2r)-2-((1s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl)-4- Methyl-1-oxopenty)lamino)-, Cyclopentyl Ester, (.alpha.s)-
49. Benzeneacetic Acid, Alpha-(((2r)-2-((1s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl)-4-methyl-1-oxopentyl)amino)-, Cyclopentyl Ester, (alphas)-
50. Cyclopentyl (2s)-2-((2r)-2-((1s)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl)-4- Methylpentanamido)-2-phenylacetate
| Molecular Weight | 406.5 g/mol |
|---|---|
| Molecular Formula | C21H30N2O6 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | 406.21038668 g/mol |
| Monoisotopic Mass | 406.21038668 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 555 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tosedostat has pleiotropic effects against a range of human tumor cell lines originating from diverse tumor types in vitro and in vivo.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Tosedostat is anti-proliferative agent which induces apoptosis in leukemic cell lines in vitro. The mechanism underlying these anti-cancer actions is unclear, particularly since normal cells are much less sensitive to the agents than transformed cells. It exerts potent anti-proliferative, pro-apoptotic and anti-angiogenic effects in vitro and shows selectivity for transformed over non-transformed cells. It inhibits a number of M1 aminopeptidase enzyme family members in vitro (eg puromycin-sensitive aminopeptidase (PSA), leukotriene A4 hydrolase (LTA4H)).