

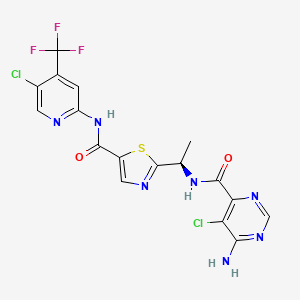

1. 2-((1r)-1-((6-amino-5-chloropyrimidine-4-carbonyl)amino)ethyl)-n-(5-chloro-4-(trifluoromethyl)pyridin-2-yl)-1,3-thiazole-5-carboxamide
2. Mln 2480
3. Mln2480
4. Tak-580
1. 1096708-71-2
2. Mln2480
3. Biib-024
4. Tak-580
5. Mln 2480
6. Mln-2480
7. Biib024
8. Tovorafenib
9. Biib 024
10. Day101
11. Tovorafenib [usan]
12. Tak-580 (mln2480)
13. Tak 580
14. Amg 2112819
15. Tak580
16. Zn90e4027m
17. 2-[(1r)-1-[(6-amino-5-chloropyrimidine-4-carbonyl)amino]ethyl]-n-[5-chloro-4-(trifluoromethyl)pyridin-2-yl]-1,3-thiazole-5-carboxamide
18. 6-amino-5-chloro-n-((1r)-1-(5-(((5-chloro-4-(trifluoromethyl)-2-pyridinyl)amino)carbonyl)-2-thiazolyl)ethyl)-4-pyrimidinecarboxamide
19. 6-amino-5-chloro-n-[(1r)-1-(5-{[5-chloro-4-(trifluoromethyl)pyridin-2-yl]carbamoyl}-1,3-thiazol-2-yl)ethyl]pyrimidine-4-carboxamide
20. 4-pyrimidinecarboxamide, 6-amino-5-chloro-n-[(1r)-1-[5-[[[5-chloro-4-(trifluoromethyl)-2-pyridinyl]amino]carbonyl]-2-thiazolyl]ethyl]-
21. Unii-zn90e4027m
22. 4-pyrimidinecarboxamide, 6-amino-5-chloro-n-((1r)-1-(5-(((5-chloro-4-(trifluoromethyl)-2-pyridinyl)amino)carbonyl)-2-thiazolyl)ethyl]-
23. 6-amino-5-chloro-n-[(1r)-1-[5-[[[5-chloro-4-(trifluoromethyl)-2-pyridinyl]amino]carbonyl]-2-thiazolyl]ethyl]-4-pyrimidinecarboxamide
24. Qop
25. Tovorafenib [inn]
26. Gtpl9977
27. Schembl4206123
28. Chembl3348923
29. Tak 580 [who-dd]
30. Day-101
31. Dtxsid70149011
32. Ex-a940
33. Chebi:167672
34. 4-pyrimidinecarboxamide, 6-amino-5-chloro-n-((1r)-1-(5-(((5-chloro-4-(trifluoromethyl)-2-pyridinyl)amino)carbonyl)-2-thiazolyl)ethyl)-
35. Bcp13797
36. Mfcd22571730
37. Nsc766906
38. Nsc800798
39. S7121
40. Who 11985
41. Zinc43202464
42. Mln 2480(biib-024)
43. Akos027323782
44. Amg2112819
45. Ccg-269746
46. Cs-0751
47. Db15266
48. Nsc-766906
49. Nsc-800798
50. Amg-2112819
51. Ncgc00346458-01
52. Ncgc00346458-02
53. Example 10da [us20090036419]
54. Hy-15246
55. P13369
56. J-690031
57. Q27295775
58. (2z,3z)-bis{amino[(2-aminophenyl)sulfanyl]methylene}succinonitrile - Ethanol (1:1)
| Molecular Weight | 506.3 g/mol |
|---|---|
| Molecular Formula | C17H12Cl2F3N7O2S |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 505.0102337 g/mol |
| Monoisotopic Mass | 505.0102337 g/mol |
| Topological Polar Surface Area | 164 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 695 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |