


1. Gsk 1120212
2. Gsk-1120212
3. Gsk1120212
4. Jtp 74057
5. Jtp-74057
6. Jtp74057
1. 871700-17-3
2. Gsk1120212
3. Mekinist
4. Gsk-1120212
5. Jtp 74057
6. Jtp-74057
7. Gsk 1120212
8. Trametinib (gsk1120212)
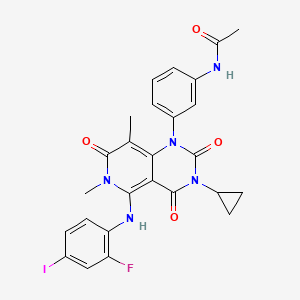
9. N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2h)-yl]phenyl]acetamide
10. Gsk212
11. Tmt212
12. Trametinib [usan]
13. Chebi:75998
14. Tmt-212
15. 33e86k87qn
16. Trametinib (usan)
17. N-(3-{3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl}phenyl)acetamide
18. N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide
19. Acetamide, N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-3,4,6,7-tetrahydro-6,8- Dimethyl-2,4,7-trioxopyrido(4,3-d)pyrimidin-1(2h)-yl)phenyl)-
20. N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide
21. Unii-33e86k87qn
22. Trametinib [usan:inn]
23. Trametinibum
24. Jtp74057
25. N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7- Tetrahydropyrido(4,3-d)pyrimidin-1(2h)-yl)phenyl)acetamide
26. N-(3-(3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido(4,3-d)pyrimidin-1(2h)-yl)phenyl)acetamide
27. N-(3-{3-cyclopropyl-5-((2-fluoro-4-iodophenyl)amino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7- Tetrahydropyrido(4,3-d)pyrimidin-1(2h)-yl}phenyl)acetamide
28. Qom
29. Trametinib [mi]
30. Trametinib (gsk1120212jtp 74057)
31. Trametinib [inn]
32. Trametinib [vandf]
33. Trametinib [who-dd]
34. Schembl170938
35. Gtpl6495
36. Gsk1120212 (trametinib)
37. Chembl2103875
38. Ex-a022
39. Bcpp000218
40. Dtxsid901007381
41. Hms3295i05
42. Hms3656j11
43. Bcp02307
44. Bdbm50531540
45. Mfcd17215075
46. Nsc758246
47. Nsc800956
48. S2673
49. Zinc43100709
50. Akos015850628
51. Am90271
52. Ccg-264976
53. Cs-0060
54. Db08911
55. Ex-5957
56. Nsc-758246
57. Nsc-800956
58. Sb16553
59. Ncgc00263180-01
60. Ncgc00263180-07
61. Ncgc00263180-14
62. Ac-25891
63. As-19382
64. Hy-10999
65. N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodo-anilino)-6,8-dimethyl-2,4,7-trioxo-pyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide
66. Ft-0688438
67. Sw218089-2
68. A25168
69. D10175
70. Gsk1120212 - Jtp-74057
71. Gsk1120212,jtp-74057, Gsk212
72. Sr-01000941589
73. A1-01871
74. J-523325
75. Q7833138
76. Sr-01000941589-1
77. Brd-k12343256-001-01-4
78. Acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2h)-yl]phenyl]-
79. N-(3-(3-cyclopropyl-5-(2-fluoro-4-iodophenylamino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl)phenyl)acetamide
80. N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2h)-yl]phe Nyl]acetamide
81. N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxopyrido[3,4-e]pyrimidin-1-yl]phenyl]acetamide
82. N-{3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2h)-yl]phenyl}ethanimidic Acid
83. N-{3-[3-cyclopropyl-5-(2-fluoro-4-iodophenylamino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydro-2h-pyrido[4,3-d]pyrimidin-1-yl]phenyl}acetamide
| Molecular Weight | 615.4 g/mol |
|---|---|
| Molecular Formula | C26H23FIN5O4 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 615.07788 g/mol |
| Monoisotopic Mass | 615.07788 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Mekinist |
| PubMed Health | Trametinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Trametinib dimethyl sulfoxide is a kinase inhibitor. The chemical name is acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4- iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl- 2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]-, compound with 1,1-sul... |
| Active Ingredient | Trametinib dimethyl sulfoxide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 1mg non-solvated parent; eq 0.5mg non-solvated parent; eq 2mg non-solvated parent |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 2 | |
|---|---|
| Drug Name | Mekinist |
| PubMed Health | Trametinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Trametinib dimethyl sulfoxide is a kinase inhibitor. The chemical name is acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4- iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl- 2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]-, compound with 1,1-sul... |
| Active Ingredient | Trametinib dimethyl sulfoxide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 1mg non-solvated parent; eq 0.5mg non-solvated parent; eq 2mg non-solvated parent |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Trametinib is indicated for the treatment of unresectable or metastatic melanoma with BRAF V600E or V600K mutations, as detected by an FDA-approved test [FDA]. In May 2018, it was approved for use with [DB08912] for the treatment of treat anaplastic thyroid cancer caused by an abnormal BRAF V600E gene.
FDA Label
* Melanoma:
Trametinib as monotherapy or in combination with dabrafenib is indicated for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation (see sections 4. 4 and 5. 1).
Trametinib monotherapy has not demonstrated clinical activity in patients who have progressed on a prior BRAF inhibitor therapy (see section 5. 1).
* Adjuvant treatment of melanoma:
Trametinib in combination with dabrafenib is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.
* Non-small cell lung cancer (NSCLC):
Trametinib in combination with dabrafenib is indicated for the treatment of adult patients with advanced non-small cell lung cancer with a BRAF V600 mutation.
Trametinib is an anticancer agent which causes apoptosis (or programmed cell death) and inhibits cell proliferation, which are both important in the treatment of malignancies.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EE01
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EE - Mitogen-activated protein kinase (mek) inhibitors
L01EE01 - Trametinib
Absorption
Trametinib is readily absorbed. When an oral administration of trametinib was given to patients with BRAF V600 mutation-positive melanoma, peak plasma concentration occurred 1.5 hours post-dose (Tmax). A single 2 mg oral dose has a bioavailability of 72%. When a dose of 2mg/day is given, the peak plasma concentration (Cmax) is 22.2 ng/mL.
Route of Elimination
80% of the dose is excreted in the feces. <20% of the dose is excreted in the urine with <0.1% of the excreted dose in the form of the parent compound.
Volume of Distribution
Apparent volume of distribution (Vd/F) = 214 L
Clearance
Apparent clearance = 4.9 L/h
Trametinib is metabolized predominantly via deacetylation alone or with mono-oxygenation or in combination with glucuronidation biotransformation pathways in vitro. Deacetylation is likely mediated by hydrolytic enzymes, such as carboxyl-esterases or amidases. The cytochrome P450 enzyme system is not involved with the metabolism of trametinib. The predominant circulating component in the plasma is the parent drug.
Elimination half-life = 3.9-4.8 days.
Trametinib is a reversible, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 _(MEK1)_ and _MEK2_ activation and of_ MEK1_ and _MEK2_ kinase activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. Trametinib helps with melanoma with the BRAF V600E or V600K as the mutation results in the constitutive activation of the BRAF pathway which includes MEK1 and MEK2.
