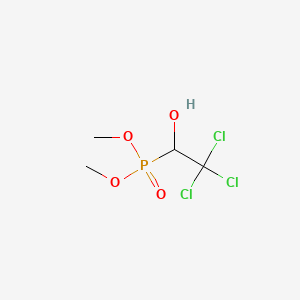

1. Bilarcil
2. Chlorofos
3. Chlorophos
4. Dipterex
5. Dylox
6. Foschlor
7. Metrifonate
8. Metriphonate
9. Neguvon
10. Ricifon
11. Trichlorphon
1. Metrifonate
2. Chlorophos
3. 52-68-6
4. Metriphonate
5. Trichlorphon
6. Methyl Chlorophos
7. Trichlorofon
8. Bilarcil
9. Chlorofos
10. Dylox
11. Chloroftalm
12. Chloroxyphos
13. Dipterex
14. Foschlor
15. Neguvon
16. Ricifon
17. Detf
18. Agroforotox
19. Chlorophthalm
20. Fliegenteller
21. Hypodermacid
22. Khloroftalm
23. Metrifonatum
24. Polfoschlor
25. Trichlorophon
26. Bovinox
27. Cekufon
28. Diptevur
29. Ditrifon
30. Forotox
31. Masoten
32. Mazoten
33. Phoschlor
34. Ritsifon
35. Sotipox
36. Volfartol
37. Votexit
38. Wotexit
39. Anthon
40. Briten
41. Briton
42. Combot
43. Danex
44. Dyrex
45. Dyvon
46. Loisol
47. Proxol
48. Soldep
49. Trinex
50. Tugon
51. (+-)-trichlorfon
52. Trichlorphon Fn
53. Dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate
54. Equino-aid
55. Foschlor R
56. Bayer L 1359
57. Chlorophosciclosom
58. Flibol E
59. Dep (pesticide)
60. Dipterex 50
61. Trichloorfon
62. Clorofos
63. Denkaphon
64. Dioxaphos
65. Foschlorem
66. Chlorak
67. Dimetox
68. Phoschlor R50
69. Dipterex Wp 80
70. Foschlor R-50
71. Metrifonato
72. Trichlorophone
73. Trichlorfon [usan]
74. Wec 50
75. Bay-a 9826
76. Bayer L 13/59
77. Nci-c54831
78. Bay 15922
79. Metifonate
80. Triclorfon
81. Dipterax
82. Neguron
83. Ent 19,763
84. Oms 800
85. Bayer-l-1359
86. 1-hydroxy-2,2,2-trichloroethylphosphonic Acid Dimethyl Ester
87. Bay-l 1359
88. (+)-trichlorfon
89. (-)-trichlorfon
90. Phosphonic Acid, (2,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester
91. Metrifonate [inn]
92. Dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate
93. Metrifonate, (+)-
94. Metrifonate, (-)-
95. Nsc-8923
96. Dbf2dg4g2k
97. 1mvy4ku98f
98. 37vcq9c0og
99. Dimethyl(2,2,2-trichloro-1-hydroxyethyl)phosphonate
100. O,o-dimethyl-1-oxy-2,2,2-trichloroethyl Phosphonate
101. Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphonate
102. Mls000069726
103. Chebi:6908
104. Chembl167150
105. O,o-dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphonate
106. O,o-dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate
107. Wec-50
108. 2,2,2-trichloro-1-dimethoxyphosphorylethanol
109. (2,2,2-trichloro-1-hydroxyethyl)phosphonic Acid Dimethyl Ester
110. O,o-dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphosaeure Ester
111. Phosphonic Acid, (1-hydroxy-2,2,2-trichloroethyl)-, Dimethyl Ester
112. Trichlorfon (usan)
113. Ent-19,763
114. O,o-dimethyl-(1-hydroxy-2,2,2-trichlorathyl)-phosphat
115. O,o-dimetil-(2,2,2-tricloro-1-idrossi-etil)-fosfonato
116. 1-hydroxy-2,2,2-trichloro-ethyle Phosphonate De Dimethyle
117. O,o-dimethyl-(2,2,2-trichlor-1-hydroxy-aethyl)phosphonat
118. Ncgc00018237-03
119. Metriphonatum
120. Britten
121. Chlorfos
122. Chlorphos
123. Ciclosom
124. Depthon
125. Leivasom
126. O,o-dimethyl-(2,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat
127. Smr000058176
128. Dimethyl (trichlorohydroxyethyl)phosphonate
129. Combot Equine
130. Dicontal Fort
131. Tugon Fly Bait
132. Dsstox_cid_1389
133. Dipterex Sl
134. Ditriphon 50
135. Dsstox_rid_76131
136. Tugon Stable Spray
137. Dsstox_gsid_21389
138. Foschlor 25
139. 26373-97-7
140. Aerol 1
141. Foschlorem [polish]
142. Trichlorphon [iso]
143. Satox 20wsc
144. Trichloorfon [dutch]
145. Trichlorfon [german]
146. Aerol 1 (pesticide)
147. Caswell No. 385
148. Vermicide Bayer 2349
149. Trichlorfon [bsi:iso]
150. Bayer 15922
151. Metrifonatum [inn-latin]
152. Phosphonic Acid, (2,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester, (+)-
153. Phosphonic Acid, (2,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester, (-)-
154. Phosphonic Acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester, (+)-
155. Phosphonic Acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester, (-)-
156. Foschlor R-50 (van)
157. Metrifonato [inn-spanish]
158. 56042-26-3
159. 56042-27-4
160. Ccris 1289
161. Hsdb 881
162. Clorofos [russian]
163. Nsc 8923
164. Einecs 200-149-3
165. Unii-dbf2dg4g2k
166. Epa Pesticide Chemical Code 057901
167. Dimethyltrichlorohydroxyethyl Phosphonate
168. Brn 1709434
169. Ertefon
170. Memobay
171. Zeltivar
172. Onefon
173. Promem
174. Ai3-19763
175. Cas-52-68-6
176. Phoschlor R.50
177. Prestwick_599
178. Foschlor R 50
179. Trichlorphon Solution
180. Dimethyl 1-hydroxy-2,2,2-trichloroethyl Phosphonate
181. O,o-dimethyl (1-oxy-2,2,2-trichloroethyl)phosphonate
182. O,o-dimethyl-1-hydroxy-2,2,2-trichloroethylphosphonate
183. Totalene (salt/mix)
184. (+/-)-trichlorfon
185. Tri Chlorfon
186. Dimethoxy-2,2,2-trichloro-1-hydroxy-ethyl Phosphine Oxide
187. Dimethoxy-2,2,2-trichloro-1-hydroxy-ethyl-phosphine Oxide
188. O,o-dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosponate
189. Starbld0043468
190. Opera_id_345
191. Metrifonate (usp/inn)
192. Trichlorfon [mi]
193. (2,2,2-trichloro-1-hydroxyethyl)-phosphonic Acid Dimethyl Ester
194. 1-hydroxy-2,2,2-trichloro-ethyle Phosphonate De Dimethyle [french]
195. O,o Dimetil 2,2,2-trichloro 1 Hidroxietil Fosfonato (portugese)
196. O,o-dimethyl-(1-hydroxy-2,2,2-trichlorathyl)-phosphat [german]
197. O,o-dimethyl-(2,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat [dutch]
198. O,o-dimethyl-(2,2,2-trichlor-1-hydroxy-aethyl)phosphonat [german]
199. O,o-dimetil-(2,2,2-tricloro-1-idrossi-etil)-fosfonato [italian]
200. Prestwick0_000051
201. Prestwick1_000051
202. Prestwick2_000051
203. Prestwick3_000051
204. Trichlorfon [iso]
205. (.+/-.)-trichlorfon
206. Trichlorfon [hsdb]
207. Trichlorfon [iarc]
208. Unii-1mvy4ku98f
209. Unii-37vcq9c0og
210. O,o-dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphonsaeure Ester [german]
211. Cid_5853
212. Bay-a-9826
213. Metrifonate [mart.]
214. Schembl15972
215. Bspbio_000201
216. Metrifonate [who-dd]
217. Metrifonate [who-ip]
218. Trichlorfon [usp-rs]
219. Spbio_002122
220. Wln: Gxggyqpo&o1&o1
221. Bpbio1_000223
222. O,2,2-trichlorathyl)-phosphat
223. O,2,2-trichloroethyl)phosphate
224. Dtxsid0021389
225. Trichlorfon [green Book]
226. Metrifonate [ep Impurity]
227. Nsc8923
228. Hms1568k03
229. Hms2095k03
230. Metrifonate [usp Impurity]
231. Pharmakon1600-01505765
232. Metrifonate [usp Monograph]
233. Hy-b1220
234. Metrifonatum [who-ip Latin]
235. Tox21_110843
236. Tox21_201607
237. Tox21_300860
238. Bdbm50286920
239. Nsc759241
240. O,o-dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphonsaeure Ester
241. Akos001499170
242. Tox21_110843_1
243. Ccg-213546
244. Cs-4845
245. Db11473
246. Nsc-759241
247. Trichlorfon 100 Microg/ml In Methanol
248. O,2,2-trichlorohydroxyethyl)phosphonate
249. Ncgc00018237-02
250. Ncgc00018237-04
251. Ncgc00018237-06
252. Ncgc00018237-07
253. Ncgc00018237-09
254. Ncgc00089822-02
255. Ncgc00089822-03
256. Ncgc00254763-01
257. Ncgc00259156-01
258. O,2,2-trichloraethyl)phosphosaeure Ester
259. O,2,2-tricloro-1-idrossi-etil)-fosfonato
260. Sbi-0206912.p001
261. Trichlorfon 100 Microg/ml In Acetonitrile
262. 0,2,2-trichloro-1-hydroxyethyl)phosphonate
263. O,2,2-trichloro-1-hydroxyethyl)phosphonate
264. O,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat
265. O,2,2-trichlor-1-hydroxy-aethyl)phosphonat
266. C07971
267. D00805
268. F21293
269. Trichlorfon, Pestanal(r), Analytical Standard
270. Q422991
271. Sr-01000000181
272. Dimethyl (1-hydroxy-2,2-trichloroethyl)phosphonate
273. Dimethyl (2,2-trichloro-1-hydroxyethyl)phosphonate
274. Dimethyl 1-hydroxy-2,2,2-trichloroethylphosphonate
275. Q-201859
276. Sr-01000000181-2
277. (2,2,2-trichloro-1-hydroxyethyl) Dimethylphosphonate
278. [(2,2-trichloro-1-hydroxyethyl) Dimethylphosphonate]
279. O,o-dimethyl (2,2,2-trichlorohydroxyethyl)phosphonate
280. Phosphonic Acid,2,2-trichloroethyl)-, Dimethyl Ester
281. Trichloro-alpha-hydroxyethyl Phosphonic Dimethyl Ester
282. 1-hydroxy-2,2-trichloro-ethyle Phosphonate De Dimethyle
283. Dimethoxy-(2,2-trichloro-1-hydroxyethyl)phosphine Oxide
284. O,o-dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphate
285. (2,2-trichloro-1-hydroxy)phosphonic Acid, Dimethyl Ester
286. (2,2-trichloro-1-hydroxyethyl)phosphonate, Dimethyl Ester
287. Dimethoxy-(2,2,2-trichloro-1-hydroxyethyl)phosphine Oxide
288. Metrifonate, European Pharmacopoeia (ep) Reference Standard
289. (1-hydroxy-2,2-trichloroethyl)phosphonic Acid, Dimethyl Ester
290. (2,2,2-trichloro-1-hydroxyethyl)phosphonate, Dimethyl Ester
291. (2,2-trichloro-1-hydroxyethyl)phosphonic Acid Dimethyl Ester
292. 1-hydroxy-2,2,2-trichloroethylphosphonate-o,o-dimethyl Ester
293. Phosphonic Acid,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester
294. (1-hydroxy-2,2,2-trichloroethyl)phosphonic Acid, Dimethyl Ester
295. 2,2-trichloro-1-hydroxyethyl)phosphonic Acid, Dimethyl Ester
296. Dimethyl (p)-(2,2,2-trichloro-1-hydroxyethyl)phosphonate
297. Dimethyl (rs)-2,2,2-trichloro-1-hydroxyethylphosphonate
298. Trichlorfon, United States Pharmacopeia (usp) Reference Standard
299. Phosphonic Acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, Dimethyl Ester
| Molecular Weight | 257.43 g/mol |
|---|---|
| Molecular Formula | C4H8Cl3O4P |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 255.922579 g/mol |
| Monoisotopic Mass | 255.922579 g/mol |
| Topological Polar Surface Area | 55.8 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 183 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anthelmintics; Cholinesterase Inhibitors; Insecticides.
National Library of Medicine's Medical Subject Headings. Trichlorfon. Online file (MeSH, 2017). Available from, as of May 24, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
Trichlorfon has been used to treat helminthiasis, including ankylostomiasis, ascariasis, trichuriasis, and creeping eruption in human. The expected pharmacological effects of the drug did appear as side effects but these effects were no more severe or frequent than those of other anthelminthics. Trichlorfon is effective for treating even Schistosoma hematobium infection.
Krieger, R. (ed.). Handbook of Pesticide Toxicology. Volume 1, 2nd ed. 2001. Academic Press, San Diego, California., p. 56
/EXPL THER/ Metrifonate (trichlorfon) is an inhibitor of acetylcholinesterase (AChE). It was used as an Alzheimer's disease (AD) drug; however, the application was withdrawn due to adverse effects. Implication of metrifonate for the antioxidant status and regulation of apoptotic processes was evaluated in the present study. Wistar rats (six per group) were exposed subcutaneously to either 60 or 120 mg/kg of body weight of metrifonate and compared with the controls treated with saline only. Cerebral cortex and liver tissues were collected from animals 40 min after exposure. Activities of AChE, glutathione reductase, glutathione-S-transferase, caspase 3, total protein level, thiobarbituric acid reactive substances, reduced glutathione level and ferric reducing antioxidant power (FRAP) were assayed in the tissue samples. Metrifonate had only lower impact on oxidative stress in the liver. Cerebral cortex tissues had decreased AChE and increased caspase 3 activities as well as the FRAP level. Owing to the novel findings, suitability of metrifonate for AD therapy is discussed.
PMID:21943232 Pohanka M et al; Toxicol Mech Methods 21 (8): 585-90 (2011)
Metrifonate is an excellent drug for the treatment of urinary schistosomiasis in areas with Schistosoma haematobium monoinfection. Toxicity apparently is negligible. Side effects due to the inhibition of acetylcholinesterase are usually scarce, light and transient in nature. At the recommended dosage of 3 time 10 mg/kg the chemotherapeutic potential of metrifonate to cure can be expected to range between 60 and 90%. Each dose of metrifonate reduces egg excretion by almost 90%. Treatment with metrifonate clearly reverses lower and upper renal tract pathology. When appropriately timed with regards to local transmission dynamics the minimal requirement to achieve 99% reduction of egg excretion may be as low as three or four doses spaced over a period of two years.
Feldmeier H, Doehring E; Acta Trop (Basel) 44 (3): 357-68 (1987)
For more Therapeutic Uses (Complete) data for Trichlorfon (13 total), please visit the HSDB record page.
/Metrifonate/ treated individuals should be free from recent exposure to insecticides that might add to the anticholinesterase effect. They also should not receive depolarizing neuromuscular blocking agents for at least 48 hr after treatment.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1133
VET: Do not use in conjunction with or within few days of (before or after) any other cholinesterase inhibitors; avoid use with phenothiazine, phenothiazine tranquilizers, arsenicals, purgatives or drugs producing purgation as side effect. Possibility of adverse reactions in heartworm infested dogs ... suggested. Do not contaminate feed or water except as in product use directions. Apply to cattle as directed only after milking. Skin sensitivity ... reported ... on pets wearing "flea collars." Avoid use in very young, debilitated, and constipated animals, or those with cirrhotic livers or acute infectious diseases.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 615
A 60% depression in red /blood/ cell /(cholinesterase)/ ChE and 80% depression in plasma ChE were accompanied by only minor side effects (nausea, vomiting, and/or diarrhea) ... .
WHO; Environ Health Criteria 132: Trichlorfon p. 74 (1992)
In ... /a/ study designed to evaluate the safety and tolerability of relatively high loading doses followed by lower maintenance doses and to determine the maximum tolerated dose of trichlorfon, groups of probable Alzheimer's disease patients were administered either 2.5 mg/kg/day for 14 days followed by 4.0 mg/kg/day for 3 days, then 2.0 mg/kg/day for 14 days or 2.5 mg/kg/day for 14 days followed by 1.5 mg/kg/day for 35 days. RBC acetylcholinesterase inhibition occurred in all groups. Moderate to severe cholinergic effects (muscle cramps, abdominal discomfort, headache, muscle weakness, generalized moderate to severe muscle cramps, weakness, inability to resume daily activities, and coordination difficulties) occurred in 6 of 8 patients given the higher doses (4.0 mg/kg/day for 3 days and 2.0 mg/kg/day for 14 days); mild to moderate cholinergic effects (gastrointestinal disturbances, muscle cramps, and light-headedness/dizziness) occurred among patients given the lower maintenance dose (1.5 mg/kg/day). /Former use/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 934
For more Drug Warnings (Complete) data for Trichlorfon (6 total), please visit the HSDB record page.
Anthelmintics
Agents that kill parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal. (See all compounds classified as Anthelmintics.)
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02B - Antitrematodals
P02BB - Organophosphorous compounds
P02BB01 - Metrifonate
Absorbed by skin.
Larranaga, M.D., Lewis, R.J. Sr., Lewis, R.A.; Hawley's Condensed Chemical Dictionary 16th Edition. John Wiley & Sons, Inc. Hoboken, NJ 2016., p. 1364
Trichlorfon ... is not readily absorbed by cotton leaves.
White-Stevens, R. (ed.). Pesticides in the Environment: Volume 1, Part 1, Part 2. New York: Marcel Dekker, Inc., 1971., p. 176
In cow, (32)P-labeled trichlorfon administered orally ... /was/ eliminated in urine, about 65% of administered dose ... /was/ excreted in 12 hr. ... 75% ... of compound of unknown structure. ... Less than 0.2% of administered dose ... in milk ... Blood contained ... max of 15.1 ug equivalent 2 hr after administration, decreased ... to about 1 ug equivalent after 24 hr.
White-Stevens, R. (ed.). Pesticides in the Environment: Volume 1, Part 1, Part 2. New York: Marcel Dekker, Inc., 1971., p. 175
The absorption, distribution, and excretion of trichlorfon in mammals is rapid: about 70-80% of a dose administered orally to mice was excreted during the first 12 hr after treatment. The biological half-life of trichlorfon was 80 min. ...3 hr after an iv injection of (14)CH3O-trichlorfon to rats, trace amounts of radioactivity were found in the liver, lungs, kidney, heart, spleen and blood.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V30 216 (1983)
For more Absorption, Distribution and Excretion (Complete) data for Trichlorfon (15 total), please visit the HSDB record page.
Trichlorfon and dichlorvos levels were followed in plasma and RBCs of 7 individuals given single oral doses of 7.5-10 mg/kg trichlorfon repeated after 2 wk for treating schistosomiases. The relationship of dichlorvos to trichlorfon in plasma and RBCs was about 1%. A biphasic curve for the elimination of trichlorfon in plasma developed; the first phase had a half-life of 0.4 to 0.6 hr, and the second phase had a half-life of about 3 hr. Clearance of trichlorfon was primarily due to formation of dichlorvos.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 929
... Trichlorfon (and its /metabolic/ product dichlorvos) is very rapidly absorbed and cleared in humans. After acute oral treatment of healthy male volunteers with a 2, 5, 7.5, or 12 mg/kg dose of trichlorfon (metrifonate), the maximum blood concentration of trichlorfon was obtained between 12 min and 2 hr and the half-life in blood was about 2 hr. The concentrations of dichlorvos ... closely followed those of trichlorphon at a constant ratio of about 1 to 100. The concentrations of trichlorfon were detectable up to 8 hr, but those of dichlorvos had fallen bellow the level of detection by then. Both plasma and RBC cholinesterases were readily inhibited and were still low after 24 hr - none of the volunteers complained of side effects (and the half-life of ... dichlorvos was about 3.8 hr).
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 929
The predominant metabolic pathway that involves cleavage of the P-C phosphonate bond that generates trichloroethanol and dimethyl phosphate which are then excreted in urine. Quantitatively minor pathways of metabolism include demethylation ... and (nonenzymatic) dehydrochlorination of dichlorvos which is rapidly metabolized to dichloroethanol and dimethyl phosphate which are then excreted in urine.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 929
Dichlorvos was detected in the brain of mice after ip injection of trichlorfon. Following dermal and intragastric application of trichlorfon to cows, the parent compound and dichlorvos were detected in the milk up to 22 days after application.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V30 216 (1983)
For more Metabolism/Metabolites (Complete) data for Trichlorfon (13 total), please visit the HSDB record page.
...The half-time of trichlorfon in human plasma is approximately 2 hours.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additives Series 45: Trichlorfon (2000). Available from, as of February 23, 2006: https://www.inchem.org/documents/jecfa/jecmono/v45je05.htm
The biological half-life of trichlorfon was 80 min /in mice/.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V30 216 (1983)
... Following a 133 mg/kg oral dose, trichlorfon was not detected ... Calculated half-lives /of dichlorvos (a nonenzymatic breakdown product of trichlorfon)/ in /rat/ blood, adipose tissue, muscle, and liver were 7, 11, 10, and 12 days, respectively.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 929
After acute oral treatment of healthy male volunteers with a 2, 5, 7.5, or 12 mg/kg dose of trichlorfon (metrifonate) ... the half-life of ... dichlorvos /in blood/ was about 3.8 hr ...
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 929