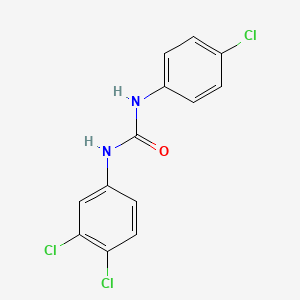

1. 3,4,4'-trichlorocarbanilide
2. Cutisan
3. Septivon
4. Septivon-lavril
5. Solubacter
6. Trichlorcarban
7. Trichlorocarbanilide
1. 101-20-2
2. 1-(4-chlorophenyl)-3-(3,4-dichlorophenyl)urea
3. 3,4,4'-trichlorocarbanilide
4. Solubacter
5. Cutisan
6. Procutene
7. Genoface
8. Trilocarban
9. Cusiter
10. N-(4-chlorophenyl)-n'-(3,4-dichlorophenyl)urea
11. Trichlorocarbanilide
12. 3,4,4'-trichlorodiphenylurea
13. Triclocarbanum
14. Urea, N-(4-chlorophenyl)-n'-(3,4-dichlorophenyl)-
15. Nsc-72005
16. Tcc
17. 3,4,4'-trichloro Carbanilide
18. 1-(3',4'-dichlorophenyl)-3-(4'-chlorophenyl)urea
19. 3,4,4-trichlorocarbanilide
20. Ent 26925
21. Cp 78416
22. N-(3,4-dichlorophenyl)-n'-(4-chlorophenyl)urea
23. Carbanilide, 3,4,4'-trichloro-
24. Nsc 72005
25. Bgg1y1ed0y
26. 3-(4-chlorophenyl)-1-(3,4-dichlorophenyl)urea
27. Trichlocarban
28. Chebi:48347
29. Nsc72005
30. Ncgc00164034-01
31. Dsstox_cid_6214
32. Dsstox_rid_78062
33. Dsstox_gsid_26214
34. Tcc Soap
35. Caswell No. 874
36. Tcc (soap Bacteriostat)
37. Triclocarban [usan:inn]
38. Triclocarbanum [inn-latin]
39. Cas-101-20-2
40. Ccris 4880
41. Hsdb 5009
42. Einecs 202-924-1
43. Unii-bgg1y1ed0y
44. Epa Pesticide Chemical Code 027901
45. Brn 2814890
46. Ai3-26925
47. (triclocarban)
48. Nipaguard Tcc
49. 9eg
50. Mfcd00013254
51. Urea,4-dichlorophenyl)-
52. Triclocarban (usp/inn)
53. Triclocarban [mi]
54. Urea-based Compound, 11
55. Triclocarban [inn]
56. 3,4'-trichlorocarbanilide
57. Ec 202-924-1
58. Triclocarban [hsdb]
59. Triclocarban [inci]
60. Triclocarban [usan]
61. 3,4'-trichlorodiphenylurea
62. Cid_7547
63. Nciopen2_008923
64. Schembl68658
65. Triclocarban [mart.]
66. Wln: Gr Dmvmr Cg Dg
67. 4-12-00-01265 (beilstein Handbook Reference)
68. Mls002415563
69. Carbanilide,4,4'-trichloro-
70. Triclocarban [usp-rs]
71. Triclocarban [who-dd]
72. 3,4,4 Inverted Exclamation Marka-trichlorocarbanilide
73. Chembl1076347
74. Dtxsid4026214
75. Bdbm25730
76. Triclocarban, Analytical Standard
77. 3,?4,?4'-?trichlorocarbanilide
78. Zinc121480
79. Hy-b1805
80. Tox21_112078
81. Tox21_202030
82. Tox21_300481
83. Triclocarban [usp Monograph]
84. S6444
85. Stk730440
86. 3,4,4'-trichlorocarbanilide, 99%
87. Akos001713711
88. Tox21_112078_1
89. Cs-7760
90. Db11155
91. Ks-5290
92. Triclocarban 100 Microg/ml In Methanol
93. Ncgc00164034-02
94. Ncgc00164034-03
95. Ncgc00164034-04
96. Ncgc00164034-05
97. Ncgc00254243-01
98. Ncgc00259579-01
99. Ac-12602
100. Smr001339078
101. 3,4,4'-trichlorocarbanilide(triclocarban)
102. Triclocarban 100 Microg/ml In Acetonitrile
103. Ft-0614114
104. T1015
105. D06223
106. E77179
107. 1-(4-chlorophenyl)-1-(3,4-dichlorophenyl)urea
108. A800352
109. Q416579
110. Sr-01000860289
111. N-(4-chlorophenyl)-n'-(3,4-dichlorophenyl)-urea
112. Q-201865
113. Sr-01000860289-2
114. Us8815951, 3-(4-chlorophenyl)-1-(3,4-dichlorophenyl)urea
115. Triclocarban, United States Pharmacopeia (usp) Reference Standard
116. 3,4,4` Trichlorocarbanilide (triclocarban), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 315.6 g/mol |
|---|---|
| Molecular Formula | C13H9Cl3N2O |
| XLogP3 | 5.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 313.978046 g/mol |
| Monoisotopic Mass | 313.978046 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 308 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiseptic, disinfectant.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1789
/EXPL THER/ The increasing use of the antimicrobial triclocarban in personal care products has resulted in concern regarding environmental pollution. Triclocarban is a potent inhibitor of soluble epoxide hydrolase (sEH). Inhibitors of sEH (sEHIs) are anti-inflammatory, anti-hypertensive and cardio-protective in multiple animal models. However, the in vivo effects anticipated from a sEHI have not been reported for triclocarban. Here we demonstrated the anti-inflammatory effects in vivo of triclocarban in a murine model. Triclocarban was employed in a lipopolysaccharide (LPS)-challenged murine model. Systolic blood pressure, plasma levels of several inflammatory cytokines and chemokine, and metabolomic profile of plasma oxylipins were determined. Triclocarban significantly reversed LPS-induced morbid hypotension in a time-dependent manner. Triclocarban significantly repressed the increased release of inflammatory cytokines and chemokine caused by LPS. Furthermore, triclocarban significantly shifted the oxylipin profile in vivo in a time-dependent manner towards resolution of inflammation as expected from a sEHI. These results demonstrated that at the doses used triclocarban is anti-inflammatory in the murine model. This study suggests that triclocarban may provide some benefits in humans in addition to its antimicrobial activities due to its potent inhibition of sEH. It may be a promising starting point for developing new low volume high value applications of triclocarban. However these biological effects also caution against the general over use of triclocarban in personal care products.
PMID:21741984 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3156297 Liu JY et al; Toxicol Appl Pharmacol 255 (2): 200-6 (2011)
Triclocarban (TCC), or 3,4,4'-trichlorocarbanilide, is an antibacterial agent used in bar and liquid soaps and body washes.
The antimicrobial mechanism underlying the bacteriostatic and bactericidal effects of triclocarban is believed to be an unspecific adsorption to cell membranes and interruption of their function. As a result, the growth of gram-positive as well as gram-negative bacteria is inhibited.
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Water Pollutants, Chemical
Chemical compounds which pollute the water of rivers, streams, lakes, the sea, reservoirs, or other bodies of water. (See all compounds classified as Water Pollutants, Chemical.)
Absorption
A human exposure study in a small group of subjects demonstrated that a portion of the TCC present in bar soaps is absorbed through the skin and is excreted in urine as N-glucuronides. Because they are produced and used in large quantities in various products, they are absorbed into the human body of the general population. The absorption of triclocarban during a human pharmacokinetic study was estimated at 0.6% of the 70 + or - 15 mg of triclocarban in the soap used. The triclocarban-N-glucuronide urine concentration varied considerably among the study subjects, and continuous daily use of the soap led to steady-state levels of excretion.
Route of Elimination
The metabolism of (14)C-TCC (3,4,4'-trichlorocarbanilide) has been investigated in humans following oral exposure to 2.2 mumol/kg. Fecal elimination (70% of dose) was complete at the 120 hour point after administration and the urinary excretion (27% of dose) was complete after 80 hours post-administration. Urinary glucuronides appear to be valuable biomarkers of triclocarban exposure.
Clearance
After a pharmacokinetic study in man, radioactivity was rapidly cleared from blood after intravenous administrations of (14)C-triclocarban in propylene glycol with a blood clearance half-life measured to be 8.6 hours.
/MILK/ ... Sprague Dawley rats were provided control, 0.2% weight/weight (w/w), or 0.5% w/w TCC-supplemented chow through a series of 3 experiments that limited exposure to critical growth periods: gestation, gestation and lactation, or lactation only (cross-fostering) to determine the susceptible windows of exposure for developmental consequences. ... The average concentration of TCC in the milk was almost 4 times that of the corresponding maternal serum levels. The results demonstrate that gestational TCC exposure does not affect the ability of dams to carry offspring to term but TCC exposure during lactation has adverse consequences on the survival of offspring although the mechanism of reduced survival is currently unknown. This information highlights the importance of evaluating the safety of TCC application in personal care products and the impacts during early life exposure.
PMID:24803507 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4527418 Kennedy RC et al; Reprod Sci 22 (1): 75-89 (2015)
The antibacterial triclocarban (TCC) concentrates in the cellular fraction of blood. Consequently, plasma levels are at least two-fold lower than the TCC amount present in blood. Utilizing whole blood sampling, a low but significant absorption of TCC from soap during showering is demonstrated for a small group of human subjects.
PMID:22273184 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3538789 Schebb NH et al; Chemosphere 87 (7): 825-7 (2012)
The route and rate of excretion by rats of the germicide (14)C-triclocarban formerly called trichlorocarbanilide, given by parenteral injection has been investigated. Blood levels based on radioactivity and by chemical determination after parenteral injection have been compared with those obtained after topical application of (14)C-triclocarban in soaps and in dimethylformamide (DMF) through occluded rat skin has been studied. Other soaps and a hand cleanser containing (14)C-triclocarban have been applied to rat skin without occlusion and the effects of duration of contact, concentration and the use of a solubilizer have been investigated. In humans, absorption of triclocarban through skin after bathing daily for 28 days has been investigated by chemical analysis of blood and urine. The data show that elimination by the rat is rapid and complete principally via the feces. Blood levels after parenteral injection are low and comparison of the radioactivity and chemical determinations suggest rapid metabolism of the Triclocarban. After application to the skin, blood levels based on (14)C-triclocarban are very low. Absorption of (14)C-triclocarban through occluded rat skin was greater from DMF than from soaps. With non-occluded rat skin, absorption from soaps was less and was dependent on concentration but independent of duration of contact. The use of a solubilizer did not increase absorption through skin. No measurable triclocarban (less than 25 ppb) was present in blood and urine samples of volunteers during or shortly after a 28-day intensive bathing regimen.
PMID:941165 Howes D, Black JG; Toxicology 6 (1): 67-76 (1976)
The metabolism and disposition of (14)C-TCC (3,4,4'-trichlorocarbanilide) have been evaluated in humans following oral exposure to 2.2 umol/kg body wt. Fecal elimination (70% of dose) was complete 120 hr after dosing and the urinary excretion (27% of dose) was completed in 80 hr. The maximum plasma level occurred 2.8 hr after dosing and was 3.7 nmol-equivalents of TCC per g of plasma (approximately 1.2 ppm). Biotransformation of TCC was rapid but did not appear to involve splitting of the basic TCC structure. The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). ...
PMID:26534 Hiles RA, Birch CG; Drug Metab Dispos 6 (2): 177-83 (1978)
For more Absorption, Distribution and Excretion (Complete) data for Triclocarban (10 total), please visit the HSDB record page.
Blood levels after parenteral injection are low and comparison of the radioactivity and chemical determinations suggest rapid metabolism of the Triclocarban. Human metabolism of TCC involves direct glucuronidation to form N- and N'- glucuronides as well as ring hydroxylation to 2'-hydroxy-TCC and 6-hydroxy-TCC, which are further metabolized to sulfate and glucuronide conjugates. In human subjects given a single oral dose of TCC, 27% of the dose was excreted in the urine within 80 hours. About 70% of the administered dose was excreted in the feces within 5 days. The major urinary metabolites were N-glucuronides (average levels, 30 ng/mL) and a major plasma metabolite was the sulfate conjugate of 2'-OH-TCC (levels ranged from 0-20 ng/mL. The maximum plasma level occurred 2.8 hr after dosing and was 3.7 nmol-equivalents of TCC per g of plasma (approximately 1.2 ppm). Biotransformation of TCC was rapid but did not appear to involve splitting of the basic TCC structure. The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half-life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile).
The metabolism and disposition of (14)C-TCC (3,4,4'-trichlorocarbanilide) have been evaluated in humans following oral exposure to 2.2 umol/kg body wt. Fecal elimination (70% of dose) was complete 120 hr after dosing and the urinary excretion (27% of dose) was completed in 80 hr. ... Biotransformation of TCC was rapid but did not appear to involve splitting of the basic TCC structure. The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). ...
PMID:26534 Hiles RA, Birch CG; Drug Metab Dispos 6 (2): 177-83 (1978)
Plant uptake and metabolism of emerging organic contaminants, such as personal-care products, pose potential risks to human health. In this study, jalapeno pepper (Capsicum annuum) plants cultured in hydroponic media were exposed to both (14)C-labeled and unlabeled triclocarban (TCC) to investigate the accumulation, distribution, and metabolism of TCC following plant uptake. The results revealed that TCC was detected in all plant tissues; after 12 weeks, the TCC concentrations in root, stem, leaf, and fruit tissues were 19.74 +/- 2.26, 0.26 +/- 0.04, 0.11 +/- 0.01, and 0.03 +/- 0.01 mg/kg dry weight, respectively. More importantly, a substantial portion of the TCC taken up by plants was metabolized, especially in the stems, leaves, and fruits. Hydroxylated TCC (e.g., 2'-OH TCC and 6-OH TCC) and glycosylated OH-TCC were the main phase I and phase II metabolites in plant tissues, respectively. Bound (or nonextractable) residues of TCC accounted for approximately 44.6, 85.6, 69.0, and 47.5% of all TCC species that accumulated in roots, stems, leaves, and fruits, respectively. The concentrations of TCC metabolites were more than 20 times greater than the concentrations of TCC in the above-ground tissues of the jalapeno pepper plants after 12 weeks; crucially, approximately 95.6% of the TCC was present as metabolites in the fruits. Consequently, human exposure to TCC through the consumption of pepper fruits is expected to be substantially higher when phytometabolism is considered.
PMID:29637774 Huynh K et al; J Agric Food Chem 66 (16): 4032-4043 (2018)
Previous studies of triclocarban suggest that its biotransformation could yield reactive metabolites that form protein adducts. Since the skin is the major route of triclocarban exposure, present work examined this possibility in cultured human keratinocytes. The results provide evidence for considerable biotransformation and protein adduct formation when cytochrome P450 activity is induced in the cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin, a model Ah receptor ligand. Since detecting low adduct levels in cells and tissues is difficult, we utilized the novel approach of accelerator mass spectrometry for this purpose. Exploiting the sensitivity of the method, we demonstrated that a substantial portion of triclocarban forms adducts with keratinocyte protein under the P450 inducing conditions employed.
PMID:22711420 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3522462 Schebb NH et al; J Biochem Mol Toxicol 26 (6): 230-4 (2012)
... After repeated oral administration of 3,4,4'-trichlorocarbanilide (TCC) ... the biliary metabolites ... were isolated and identified. The major TCC biliary metabolite was found to be 2'-hydroxy-TCC. This compound was isolated mainly from the nonconjugated and the glucuronide fractions. Other metabolites present in substantial quantities were 6-hydroxy-TCC and 2',6-dihydroxy-TCC mainly as glucuronides and 3'-hydroxy-TCC mainly as the sulfate conjugate. Small amounts of 3',6-dihydroxy-TCC were isolated from each of the fractions. No unchanged TCC was found in the bile. Only traces of other metabolites were found, and no N-hydroxylated products were observed. ...
PMID:15808 Jeffcoat AR Et Al; Drug Metab Dispos 5 (2): 157-66 (1977)
For more Metabolism/Metabolites (Complete) data for Triclocarban (7 total), please visit the HSDB record page.
10 hours
The metabolism and disposition of (14)C-TCC (3,4,4'-trichlorocarbanilide) have been evaluated in humans following oral exposure to 2.2 umol/kg body wt. ... The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). ...
PMID:26534 Hiles RA, Birch CG; Drug Metab Dispos 6 (2): 177-83 (1978)
... Radioactivity was rapidly cleared from blood after intravenous administrations of (14)C-triclocarban in propylene glycol with a blood clearance half-life of 8.6 hours. About 54% of the dose was excreted in the feces and 21% of the dose in the urine with a urinary elimination half-life of ten hours. ...
PMID:1109279 Scharpf LG Jr et al; Arch Environ Health 30 (1): 7-14 (1975)
Triclocarban is a triclosan analog with an antibacterial activity. Triclocarban exerts its effect by inhibiting the activity of _enoyl-(acyl-carrier protein) (ACP) reductase_, which is ubiquitously distributed in bacteria, fungi and various plants. ACP reductase catalyzes the last step in each cycle of fatty acid elongation in the type II fatty acid synthase systems. As a result, this agent interrupts cell membrane synthesis and leads to bacterial growth inhibition.
As a carbanilide, /triclocarban/ can be classified according to its antimicrobial mechanism as a membrane active compound. The mode of action can be described as unspecific adsorption to cell membranes, interruption of the function of interstitial proteins and/or loss of the semipermeability of the membrane, with discharge of ions and organic molecules. Bacteriostatic or bactericidal effects occur dependent on the concentration. In its standard application concentrations, triclocarban inhibits primarily the growth of gram-positive bacteria, but also that of gram-negative bacteria. Unlike antibiotics, membrane-active antimicrobial substances such as triclocarban are effective within a short period of time.
European Commission; Health & Consumer Protection Directorate-General; Scientific Committee on Consumer Products (SCCP), Opinion on Triclocarban for Other Uses Than as a Preservative, p4 (June 2005). Available from, as of May 8, 2018: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_016.pdf