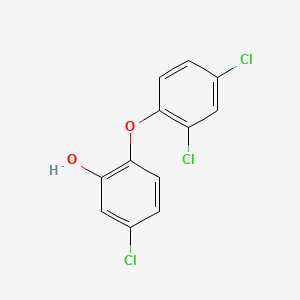

1. 2,4,4'-trichloro-2'-hydroxydiphenyl Ether
2. 2-hydroxy-2',4,4'-trichlorodiphenyl Ether
3. Aquasept
4. Clearasil Daily Face Wash
5. Cliniclean
6. Dp300, Irgasan
7. Irgasan Dp 300
8. Irgasan Dp300
9. Irgasan-dp300
10. Manusept
11. Microshield T
12. Oxy Skin Wash
13. Phisohex
14. Sapoderm
15. Ster Zac Bath Concentrate
16. Ster-zac Bath Concentrate
17. Sterzac Bath Concentrate
18. Tersaseptic
19. Trisan
1. 3380-34-5
2. 5-chloro-2-(2,4-dichlorophenoxy)phenol
3. Irgasan
4. 2,4,4'-trichloro-2'-hydroxydiphenyl Ether
5. Cloxifenolum
6. Triclosanum
7. Irgasan Dp300
8. Phenol, 5-chloro-2-(2,4-dichlorophenoxy)-
9. Stri-dex Cleansing Bar
10. Ch 3565
11. 5-chloro-2-(2,4-dichloro-phenoxy)-phenol
12. 2,4,4'-trichloro-2'-hydroxy Diphenyl Ether
13. Chebi:164200
14. Ether, 2'-hydroxy-2,4,4'-trichlorodiphenyl
15. Phenyl Ether, 2'-hydroxy-2,4,4'-trichloro-
16. Chembl849
17. Nsc-759151
18. Ch-3565
19. 4nm5039y5x
20. Dndi1246774
21. Triclosan 10 Microg/ml In Cyclohexane
22. Tcl
23. Ncgc00159417-02
24. Ncgc00159417-05
25. Ncgc00159417-06
26. Stri-dex Face Wash
27. Aquasept
28. Sapoderm
29. Lexol 300
30. Dsstox_cid_12498
31. Dsstox_rid_78958
32. Dsstox_gsid_32498
33. Triclosanum [inn-latin]
34. Dp-300
35. Caswell No. 186a
36. Cloxifenol
37. Smr000471847
38. Cas-3380-34-5
39. Hsdb 7194
40. Triclosan (usp/inn)
41. Sr-01000762974
42. Einecs 222-182-2
43. Epa Pesticide Chemical Code 054901
44. Brn 2057142
45. Sterzac
46. Stri-dex Cleansing Bar (tn)
47. Unii-4nm5039y5x
48. Ccris 9253
49. Triclosan Usp
50. 1nhg
51. Triclosan [usan:usp:inn:ban]
52. Triclosan; Irgasan
53. Mfcd00800992
54. Irgasan Dp 30
55. Tccp
56. Neostrata Antibacterial Facial Cleanser
57. 3p9t
58. 4w9n
59. Triclosan [inn]
60. Triclosan [mi]
61. Triclosan [hsdb]
62. Triclosan [inci]
63. Triclosan [usan]
64. Triclosan [vandf]
65. Epitope Id:119683
66. Triclosan [mart.]
67. Schembl3269
68. Triclosan [usp-rs]
69. Triclosan [who-dd]
70. Mls001066347
71. Mls001074876
72. Mls001335937
73. Mls001335938
74. Bdbm8726
75. Zinc2216
76. Triclosan [orange Book]
77. Dtxsid5032498
78. Triclosan [usp Monograph]
79. Hms2093l17
80. Hms2270m06
81. Hms3259k19
82. Hms3715h11
83. Hms3872n03
84. Pharmakon1600-01505465
85. Irgasan, >=97.0% (hplc)
86. Hy-b1119
87. Tox21_111648
88. Tox21_400079
89. Dp 300
90. Nsc759151
91. S4541
92. Stl451034
93. Triclosan 100 Microg/ml In Methanol
94. Akos015850380
95. Tox21_111648_1
96. Ccg-213459
97. Ch-3635
98. Cs-4718
99. Db08604
100. Ks-5356
101. Nc00516
102. Nsc 759151
103. Colgate Total Component Triclosan
104. Ncgc00159417-03
105. Ncgc00159417-04
106. Ncgc00159417-07
107. Ncgc00159417-08
108. 2,4,4'-trichloro-2'-hydroxydiphenylethe
109. Ac-10667
110. 5-chloro-2-(2, 4-dichlorophenoxy)phenol
111. Sbi-0206807.p001
112. Triclosan Component Of Colgate Total
113. 5-chloro-2-(2,4-di-chloro-phenoxy)-phenol
114. Ft-0609773
115. T1872
116. D06226
117. D70549
118. S00100
119. 5-chloro-2-(2,4-dichlorophenoxy)phenol, 97%
120. Ab00698074_08
121. Triclosan, Antibiotic For Culture Media Use Only
122. 380t345
123. A821950
124. Q408646
125. 2-[2,4-bis(chloranyl)phenoxy]-5-chloranyl-phenol
126. Q-201866
127. Sr-01000762974-2
128. Sr-01000762974-3
129. Brd-k41731458-001-04-5
130. Brd-k41731458-001-09-4
131. Triclosan, Certified Reference Material, Tracecert(r)
132. F2173-0825
133. Triclosan, United States Pharmacopeia (usp) Reference Standard
134. Triclosan, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 289.5 g/mol |
|---|---|
| Molecular Formula | C12H7Cl3O2 |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 287.951163 g/mol |
| Monoisotopic Mass | 287.951163 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 252 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents, Local; Fatty Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings. Triclosan. Online file (MeSH, 2017). Available from, as of November 14, 2017: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Triclosan is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of November 14, 2017: https://clinicaltrials.gov/
Control of meticillin-resistant Staphylococcus aureus (MRSA) infection in surgical units has been achieved by procedures including handwashing and bathing with triclosan.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 1665
Triclosan is a chlorinated bisphenol antiseptic, effective against Gram-positive and most Gram-negative bacteria but with variable or poor activity against Pseudomonas spp. It is also active against fungi. It is used in soaps, creams, and solutions in concentration of up to 2% for disinfection of the hands and wounds and for disinfection of the skin prior to surgery, injections, or venepuncture. It is also used in oral hygiene products and in preparations for acne.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 1665
For more Therapeutic Uses (Complete) data for Triclosan (7 total), please visit the HSDB record page.
Triclosan is used in a variety of common household products, including soaps, mouthwashes, dish detergents, toothpastes, deodorants, and hand sanitizers. It is also used in health care settings in surgical scrubs and personnel hand washes.
Fatty Acid Synthesis Inhibitors
Compounds that interfere with FATTY ACID SYNTHASE resulting in a reduction of FATTY ACIDS. This is a target mechanism in humans of some ANTINEOPLASTIC AGENTS and ANTI-OBESITY AGENTS and of some ANTI-INFECTIVE AGENTS which interfere with CELL WALL and CELL MEMBRANE formation. (See all compounds classified as Fatty Acid Synthesis Inhibitors.)
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AE - Phenol and derivatives
D08AE04 - Triclosan
D - Dermatologicals
D09 - Medicated dressings
D09A - Medicated dressings
D09AA - Medicated dressings with antiinfectives
D09AA06 - Triclosan
Absorption
A study conducted in 2000 demonstrated that low amounts of triclosan can be absorbed through skin and can enter the bloodstream. [PMID: 10722890] Triclosan is rapidly absorbed and distributed in the human body (Sandborgh-Englund et al., 2006). Maximum concentrations are reached within three hours after oral intake. However, the metabolism and excretion of the compound is fast.
Route of Elimination
In one study, after in vivo topical application of a 64.5mM alcoholic solution of [(3)H]triclosan to rat skin, 12% radioactivity was recovered in the faeces, 8% in the carcass 1% in the urine, 30% in the stratum corneum and 26% was rinsed from the skin surface at 24 hours after application. [PMID: 10722890]
The number of personal hygiene products containing triclosan has increased rapidly during the last decade, and triclosan is one of the most common antibacterial compounds used in dentifrices today. However, the extent of triclosan exposure has not yet been well described. The potential risks of generating triclosan-resistant pathogenic microorganisms or of the selection of resistant strains are some areas of concern. The aim of the present study was to (1) obtain information on baseline levels of triclosan in plasma and urine, and (2) study the pharmacokinetic pattern of triclosan after a single-dose intake. Ten healthy volunteers were exposed to a single oral dose of 4 mg triclosan by swallowing an oral mouthwash solution. Triclosan in plasma and urine was followed before and up to 8 days after exposure. Triclosan levels in plasma increased rapidly, with a maximum concentration within 1 to 3 hr, and the terminal plasma half-life was 21 hr. The major fraction was excreted within the first 24 hr. The accumulated urinary excretion varied between the subjects, with 24 to 83% of the oral dose being excreted during the first 4 d after exposure. In conclusion, triclosan appears to be readily absorbed from the gastrointestinal tract and has a rapid turnover in humans. The high lipid solubility of the substance gives rise to questions regarding distribution properties and accumulation. The findings of the present study form a basis for greater understanding of the toxicokinetic properties of triclosan in humans.
PMID:16952905 Sandborgh-Englund G et al; J Toxicol Environ Health A 69 (20): 1861-73 (2006)
/MILK/ Triclosan amounts in breast milk were reported to range from <20 to 300 ug/kg lipid in one study and <5 to 2100 ug/kg lipid in another. In a study that compared triclosan levels in women who used triclosan-containing products with those who did not, levels in breast milk were 0.022 to 0.95 ug/kg lipid compared to 0.018 to 0.35 ug/kg lipid, respectively.
Cosmetic Ingredient Review; Final Report on Triclosan p.9-10 (December 14, 2010). Available from, as of November 13, 2017: https://www.cir-safety.org/ingredients
Oral and dermal routes (humans and rodents): Triclosan glucuronide is predominantly excreted in the urine, and triclosan is predominantly excreted in the feces. Triclosan that is administered orally and dermally is excreted in greater concentrations in the urine than in the feces in humans, hamsters, rabbits, and monkey. In rats, mice, and dog, the reverse is true. Up to 87% of triclosan that is administered to humans (by an unspecified route) is excreted in the urine, most of it within 72 hr after dose.
Cosmetic Ingredient Review; Final Report on Triclosan p.9 (December 14, 2010). Available from, as of November 13, 2017: https://www.cir-safety.org/ingredients
The percutaneous absorption of unlabeled triclosan was investigated in a pilot study and a 90-day study with infant Rhesus monkeys. In the pilot study, triclosan was detected in all blood samples following a single dermal exposure to a soap solution containing triclosan (1 mg/mL, 0.1%), with blood levels detected up to 24 hours, and peak levels observed at 8 to 12 hours. In the 90-day study, only the glucuronide and sulfate conjugates were detected in blood samples, the glucuronide predominating in the early blood samples (Days 1 to 2), and triclosan sulfate predominating in all subsequent blood samples (samples were taken daily for the 90-day duration of the study). Triclosan was excreted in the urine primarily as the glucuronide conjugate, but was excreted in the feces primarily in the free or unconjugated form. Low levels of triclosan were detected in tissues. The results of this monkey study indicate that triclosan was absorbed percutaneously following 90 days of daily washing with 15 mL of soap (1 mg triclosan/mL) and that the proportion of plasma glucuronide and sulfate conjugates altered following chronic administration.
European Commission; Scientific Committee on Consumer Products (SCCP) - Opinion on Triclosan; SCCP/1192/08 p.20 (January 21, 2009). Available from, as of November 13, 2017: https://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/sccp_opinions_en#6
For more Absorption, Distribution and Excretion (Complete) data for Triclosan (14 total), please visit the HSDB record page.
Triclosan is prone to phase II metabolism via sulfotransferase and glucuronosyltransferase enzymes (Wang et al., 2004). In humans the resulting conjugates are excreted primarily in urine (Sandborgh-Englund et al., 2006).
Oral and dermal routes (humans and rodents): Triclosan absorbed from the gastrointestinal tract undergoes extensive first-pass metabolism, which primarily involves glucuronide and sulfate conjugation. In both humans and rodents, at high triclosan plasma concentrations, metabolism shifts from the generation of predominantly glucuronide conjugates to sulfate-conjugates. The bioavailability of unconjugated triclosan may be limited after oral exposure because of triclosan's extensive first-pass metabolism. Triclosan is also metabolized to its glucuronide and sulfate conjugates by the skin.
Cosmetic Ingredient Review; Final Report on Triclosan p.9 (December 14, 2010). Available from, as of November 13, 2017: https://www.cir-safety.org/ingredients
Triclosan has been widely used as a disinfectant in human health care products. Although this particular chemical is less toxic, its biotransformation products might have toxicity to human. Therefore, understanding the pharmacokinetics and metabolism of triclosan in animal and human body is important. Plasma samples from SD rats collected after the oral administration of 5 mg/kg triclosan were analyzed ... . The pharmacokinetic data of triclosan in the rats were presented including the half time of elimination that was (48.5 +/- 10.5) hr, indicating that the elimination of triclosan in the rat was slow. Two hydroxylated and sulfonated triclosan, one glucuronidated triclosan, and one sulfonated triclosan were identified in the rat plasma samples.
PMID:20073210 Wu J et al; Se Pu 27 (5): 724-30 (2009)
...Irgasan DP 300 is excreted unchanged in feces and urine (partly conjugated) but is also hydroxylated to five different monohydroxy metabolites which were found in urine; three of these were also present in feces.
PMID:433312 Tulp MTM et al; Xenobiotica 9 (2): 65-77 (1979)
Triclosan has known human metabolites that include (2S,3S,4S,5R)-6-[5-chloro-2-(2,4-dichlorophenoxy)phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid and Triclosan sulfate.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The terminal plasma half life of triclosan is 21 h (Sandborgh-Englund et al., 2006).
Measurements of the oral retention and pharmacokinetics of (3)H-triclosan delivered from a toothpaste containing 0.2% (3)H-triclosan were made in 12 human volunteers (aged 19-37 yr). ... After use of a one g quantity of the toothpaste, 36.3+-1.4% of the (3)H-triclosan was retained. ... The saliva decay curve for (3)H-triclosan was consistent with a 2 phase model with half life values of 0.45 hr and 2.42 hr, respectively. ...
PMID:3593627 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1386194 Gilbert RJ et al; Br J Clin Pharmacol 23: 579-583 (1987)
... Ten healthy volunteers were exposed to a single oral dose of 4 mg triclosan by swallowing an oral mouthwash solution. Triclosan in plasma and urine was followed before and up to 8 days after exposure. Triclosan levels in plasma increased rapidly, with a maximum concentration within 1 to 3 hr, and the terminal plasma half-life was 21 hr. ...
PMID:16952905 Sandborgh-Englund G et al; J Toxicol Environ Health A 69 (20): 1861-73 (2006)
The blood half-life of (14)C-triclosan during the beta-phase was 8.8 +/- 0.6 hr and the blood clearance rate was 77.5 +/- 11.3 mL/kg/hr /after injection either via the femoral vein, (iv, 5 mg/kg in polyethylene glycol-400) or into the vaginal orifice (ivg, 5 mg/kg in corn oil)/ .
PMID:422939 Siddiqui WH et al; J Environ Pathol Toxicol 2 (3): 861-71 (1979)
Plasma samples from SD rats collected after the oral administration of 5 mg/kg triclosan were analyzed ... . The pharmacokinetic data of triclosan in the rats were presented including the half time of elimination that was (48.5 +/- 10.5) hr, indicating that the elimination of triclosan in the rat was slow. Two hydroxylated and sulfonated triclosan, one glucuronidated triclosan, and one sulfonated triclosan were identified in the rat plasma samples.
PMID:20073210 Wu J et al; Se Pu 27 (5): 724-30 (2009)
Triclosan is a biocidal compound with multiple targets in the cytoplasm and membrane. At lower concentrations, however, triclosan appears bacteriostatic and is seen to target bacteria mainly by inhibiting fatty acid synthesis. Triclosan binds to enoyl-acyl carrier protein reductase enzyme (ENR). This complex has increased affinity for NAD+ and forms a ternary complex. This complex is unable to participate in fatty acid synthesis, weakening the cell membrane and causing cell death. Humans do not have an ENR enzyme, and thus are not affected.
Triclosan (TCS) exposure has widely adverse biological effects such as influencing biological reproduction and endocrine disorders. While some studies have addressed TCS-induced expression changes of miRNAs and their related down-stream target genes, no data are available concerning how TCS impairs miRNA expression leading us to study up-stream regulating mechanisms. Four miRNAs (miR-125b, miR-205, miR-142a and miR-203a) showed differential expression between TCS-exposure treatments and the control group; their functions mainly involved fatty acid synthesis and metabolism. TCS exposure led to the up-regulation of mature miR-125b that was concomitant with consistent changes in pri-mir-125b-1 and pri-mir-125b-3 among its 3 pri-mir-125bs. Up-regulation of miR-125b originated from direct shear processes involving the two up-regulated precursors, but not pri-mir-125b2. Increased expression of pri-mir-125b-1 and pri-mir-125b-3 resulted from nfe2l2- and c/ebpa-integration with positive control elements of promoters for the two precursors. The overexpression of transcriptional factors, nfe2l2 and c/ebpa, initiated the promoter activity for the miR-125b precursor. CpG islands and Nfe2l2 were involved in constitutive expression of mir-125b-1 and mir-125b-3. The activities of two promoter regions, -487 to -1bp for pri-mir-125b1 and -1327 to +14bp for pri-mir-125b-3 having binding sites for NFE2 and Nfe2l2/MAF:NFE2, were higher than other regions, further demonstrating that the transcriptional factor Nfe2l2 was involved in the regulation of pri-mir-125b1 and pri-mir-125b-3. TCS's estrogen activity resulted from its effects on GPER, a novel membrane receptor, rather than the classical ERa and ERbeta. These results explain, to some extent, the up-stream mechanism for miR-125b up-regulation, and also provide a guidance to future mechanistic study on TCS-exposure.
PMID:29121543 Lin J et al; Aquat Toxicol 193: 256-267 (2017)