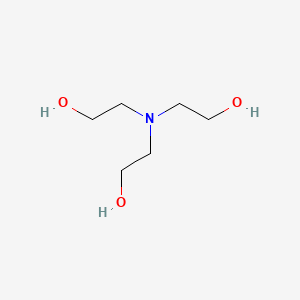

1. 2,2',2''-nitrilotriethanol
2. Triethanolamine Acetate
3. Triethanolamine Citrate
4. Triethanolamine Citrate (1:1)
5. Triethanolamine Copper Salt
6. Triethanolamine Hydrochloride
7. Triethanolamine Iodohydrate
8. Triethanolamine Maleate
9. Triethanolamine Phosphate
10. Triethanolamine Sulfate
11. Triethanolamine Sulfate (2:1)
12. Triethanolamine Sulfite (1:1)
13. Triethanolamine Tartrate (1:1), (r-(r*,r*))-isomer
14. Triethanolamine Titanium Salt
15. Triethanolammonium Chloride
16. Trolamine
1. Trolamine
2. 102-71-6
3. 2,2',2''-nitrilotriethanol
4. Sterolamide
5. Tris(2-hydroxyethyl)amine
6. Daltogen
7. Nitrilotriethanol
8. Triethylolamine
9. Trihydroxytriethylamine
10. Thiofaco T-35
11. Triethanolamin
12. Sting-kill
13. Ethanol, 2,2',2''-nitrilotris-
14. Tri(hydroxyethyl)amine
15. Tris(beta-hydroxyethyl)amine
16. Nitrilo-2,2',2''-triethanol
17. Sodium Isa
18. Alkanolamine 244
19. Teoa
20. Tea (amino Alcohol)
21. 2,2',2''-nitrilotris(ethanol)
22. Nitrilotris(ethanol)
23. 2-[bis(2-hydroxyethyl)amino]ethanol
24. Tris(hydroxyethyl)amine
25. Triethanolamin-ng
26. H3tea
27. Triaethanolamin-ng
28. Trolamine [inn]
29. 2,2',2-nitrilotriethanol
30. N(ch2ch2oh)3
31. Mobisyl
32. 2,2',2''-nitrilotrisethanol
33. Trola
34. Triethanolamine Homopolymer
35. 2,2',2''-trihydroxytriethylamine
36. Poly(triethanolamine) Ether
37. Trihydroxyethylamine
38. Trolamine (nf)
39. Trolamine [nf]
40. Mfcd00002855
41. Nsc 36718
42. Mobisy (tn)
43. Triethylamine, 2,2',2''-trihydroxy-
44. Triethanolamine Condensate Polymer
45. Ethanol, 2,2',2''-nitrilotri-
46. Ethanol, 2,2',2''-nitrilotris-, Homopolymer
47. Nsc-36718
48. Nciopen2_004601
49. 2,2',2"-nitrilotriethanol
50. 2-[bis(2-hydroxyethyl)amino]ethan-1-ol
51. 9o3k93s3tk
52. 2,2',2-nitrilotris(ethanol)
53. Chebi:28621
54. 2,2',2''-nitrilotris[ethanol]
55. Ncgc00159411-02
56. Dsstox_cid_1392
57. Tri-beta-hydroxy Ethylamine
58. Dsstox_rid_76134
59. Dsstox_gsid_21392
60. 64114-46-1
61. Caswell No. 886
62. Biafine
63. Tris(2-hydroxyethyl) Amine
64. Cas-102-71-6
65. Ccris 606
66. Hsdb 893
67. Triethanol Amine
68. Einecs 203-049-8
69. 2,2,2-nitrilotriethanol
70. Epa Pesticide Chemical Code 004208
71. Unii-9o3k93s3tk
72. Ethanolamines
73. 2,2',2'-nitrilotriethanol
74. Cerumenex
75. Alkano
76. Mobisy
77. Ai3-01140
78. Ethanol, 2,2',2'-nitrilotris-
79. Triethanolamine 85%
80. Triethanolamine 99%
81. Mobisyl (salt/mix)
82. Triethanolamine, Usp
83. Trolamine [ii]
84. Trolamine [hsdb]
85. Tris(b-hydroxyethyl)amine
86. Trolamine [vandf]
87. 2,2''-nitrilotriethanol
88. Bmse000379
89. Trolamine [mart.]
90. Ec 203-049-8
91. Nitrilo-2,2''-triethanol
92. Schembl1146
93. Trolamine [who-dd]
94. Triethanolamine [mi]
95. Oprea1_614203
96. Tris-(2-hydroxyethyl)-amine
97. Wln: Q2n2q2q
98. Tris(beta -hydroxyethyl)amine
99. 2,2''-nitrilotris[ethanol]
100. Bidd:er0261
101. Triethanolamine [iarc]
102. Triethanolamine [inci]
103. Ethanol,2',2''-nitrilotri-
104. Triethanolamine [vandf]
105. Tris(.beta.-hydroxyethyl)amine
106. Chembl446061
107. Ethanol,2',2''-nitrilotris-
108. Triethanolamine, Lr, >=99%
109. 2,2',2'-nitrilotris-ethanol
110. 2,2'2''-nitrilotris-ethanol
111. Trolamine [ep Impurity]
112. 2,2', 2''-nitrilotriethanol
113. 2,2',2quot -nitrilotriethanol
114. Dtxsid9021392
115. Trolamine [ep Monograph]
116. 2,2',2''-nitrilotri-ethanol
117. 2,2',2''-nitrilotris-ethanol
118. Nitrilo-2,2',2quot -triethanol
119. Triethanolamine, P.a., 99.0%
120. Triethanolamine, Ar, >=99.5%
121. Zinc896409
122. Triethanolamine 99% Reagent Grade
123. Triethylamine,2',2''-trihydroxy-
124. Adal1017515
125. Hy-b1809
126. Nsc36718
127. Tox21_113166
128. Tox21_202062
129. Tox21_300527
130. Triethanolamine, >=99.0% (gc)
131. 2,2',2''-trihydroxy-triethylamine
132. Stl264185
133. Triethanolamine, Reagent Grade, 98%
134. Akos000119997
135. Cs-5859
136. Db13747
137. 637-39-8 (unspecified Hydrochloride)
138. Smp2_000190
139. Tea, 0.2m Buffer Solution, Ph 7.0
140. Tea, 0.2m Buffer Solution, Ph 8.0
141. Ncgc00159411-03
142. Ncgc00159411-04
143. Ncgc00159411-05
144. Ncgc00159411-06
145. Ncgc00254460-01
146. Ncgc00259611-01
147. 7376-31-0 (unspecified Sulfate Salt)
148. Bp-21029
149. Ls-13235
150. Triethanolamine, Usp, 99.0-107.4%
151. 7376-31-0 (unspecified Sulphate Salt)
152. Triethanolamine, Bioultra, >=99.5% (gc)
153. Triethanolamine, Analytical Reference Material
154. Triethanolamine, Saj First Grade, >=98.0%
155. C06771
156. D00215
157. Triethanolamine, Jis Special Grade, >=98.0%
158. Triethanolamine, Puriss. P.a., >=99% (gc)
159. Triethanolamine, Vetec(tm) Reagent Grade, 97%
160. Q424314
161. Sr-01000944572
162. J-525067
163. Sr-01000944572-1
164. B886ef22-aca3-4597-9c73-a087e02f54a7
165. Trolamine, European Pharmacopoeia (ep) Reference Standard
166. Trolamine, United States Pharmacopeia (usp) Reference Standard
167. Triethanolamine, Puriss., Meets Analytical Specification Of Nf, >=99% (gc)
168. Trolamine, Pharmaceutical Secondary Standard; Certified Reference Material
169. Triethanolamine, Pharmagrade, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production.
| Molecular Weight | 149.19 g/mol |
|---|---|
| Molecular Formula | C6H15NO3 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 149.10519334 g/mol |
| Monoisotopic Mass | 149.10519334 g/mol |
| Topological Polar Surface Area | 63.9 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 55.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ This multicentered phase III trial was designed to compare an emulsion containing trolamine against the usual supportive care within each participating institution for patients with head and neck cancer undergoing radiation therapy. Patients with biopsy-proven squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx were randomly assigned to one of the following treatments: prophylactic trolamine emulsion, interventional trolamine emulsion, or declared institutional preference. The primary outcome was the reduction in grade 2 or higher skin toxicity, as per National Cancer Institute Common Toxicity Criteria version 2.0. Secondary outcomes included patient-reported quality of life (QOL). From October 2000 to April 2002, 547 patients from 51 institutions were entered onto the trial. The average age was 59 years. Patients were predominately male (79%) and most continued to use tobacco products (52%). The rates of grade 2 or higher radiation dermatitis were 79%, 77%, and 79% in the prophylactic, interventional, and institutional preference arms of the study, respectively. No significant differences in QOL were found. The results of this trial demonstrate no advantage for the use of trolamine in reducing the incidence of grade 2 or higher radiation dermatitis or improving patient-reported QOL. The use of 15 different local standards of care highlights the need to continue research that will result in evidence-based recommendations to reduce the burden of radiation dermatitis.
PMID:1664851 Elliott EA et al; J Clin Oncol 24 (13): 2092-7 (2006)
Triethanolamine salicylate has also been used as a nonsteroidal anti-inflammatory. Triethanolamine USP is also used as a pharmaceutical adjuvant or alkalizing agent, and in combination with a fatty acid (e.g., oleic acid, stearic acid) as an emulsifier (the triethanolamine soap formed lowers the surface tension of the aqueous phase). /Triethanolamine salicylate/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 785
2= Slightly toxic: Probable oral lethal dose (human) 5-15 g/kg, between 1 pint & 1 qt for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-106
Trolamine is used as an alkalizing agent, surfactant, and counter-ion in cosmetic and pharmaceutical formulations. It is not considered to be an active pharmacological ingredient and so has no official indication.
Acts as a surfactant or alkalizing agent to aid in emulsification and solubilizing of compounds or in raising the pH of a solution
D - Dermatologicals
D03 - Preparations for treatment of wounds and ulcers
D03A - Cicatrizants
D03AX - Other cicatrizants
D03AX12 - Trolamine
Absorption
Dermal absorption of trolamine increases with the dose. This has been found to range from 19-28% in rats with doses of 68-276 mg/kg in 190 L of acetone without occlusion and from 60-80% in mice with doses of 79-1120 mg/kg in the same volume of acetone.
Route of Elimination
When orally administered to rats, the 53% of the trolamine dose was found to be excreted in the urine and 20% in the feces. 98% was excreted in the urine with intravenous administration.
The elimination of 14(C)triethanolamine from the blood of mice administered 1.0 mg/kg bw iv showed first-order biphasic kinetics with a rapid (0.58-hr half life) and a slow phase (10.2-hr half-life). The slow phase half-lives for elimination of triethanolamine in mice after dermal exposure to 1000 and 2000 mg/kg bw in acetone were 9.7 hr and 18.6 hr. Skin absorption rates (as blood concentration-time curves) after dermal application of aqueous and neat 14(C)triethanolamine to mouse skin (2000 mg/kg bw, enclosed by a glass ring) showed no significant change with the use of water as the vehicle.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V77 389 (2000)
In a dermal pharmacokinetic study, (14)C-triethanolamine was absorbed more slowly and less extensively in F344 rats than in C3H/HeJ mice. 48 hr after dermal application of (14)C-triethanolamine to mice (1,000 mg/kg dose), about 60% of the radioactivity was recovered from the urine and about 20% was recovered in the feces; less than 10% of the radioactivity was found in skin at the site of application. It was concluded that triethanolamine does not undergo extensive biotransformation in mice, since greater than 95% of the radioactivity recovered from the urine was identified as the parent compound.
Snyder, R. (ed.). Ethel Browning's Toxicity and Metabolism of Industrial Solvents. 2nd ed. Volume II: Nitrogen and Phosphorus Solvents. Amsterdam-New York-Oxford: Elsevier, 1990., p. 443
Triethanolamine was rapidly absorbed in orally dosed rats, and subsequently excreted mainly as unchanged parent compound in the urine. 24 hr after oral administration of triethanolamine (single dose of 2-3 mg/kg), 53% and 20% of the administered dose was recovered as the parent compound in the urine and feces, respectively.
Snyder, R. (ed.). Ethel Browning's Toxicity and Metabolism of Industrial Solvents. 2nd ed. Volume II: Nitrogen and Phosphorus Solvents. Amsterdam-New York-Oxford: Elsevier, 1990., p. 443
...After single oral administration to male rats, the excretion ratios of unchanged /triethanolamine/ in the urine and feces for one day were 53% and 20% of the dose, respectively.
Kohri N et al; Arch Pract Pharm 42: 342-348 (1982)
For more Absorption, Distribution and Excretion (Complete) data for TRIETHANOLAMINE (8 total), please visit the HSDB record page.
Trolamine is excreted mostly as the unchanged compound. No diethanolamine or ethanolamine has been found. Very small amounts of trolamine glucuronide have been detected but not quantified.
...N-nitrosodiethanolamine, known carcinogen and mutagen, ...may not be the main mutagenic product.
PMID:100210 Hoshino H, Tanooka H; Cancer Res 38 (11): 3918-21 (1978)
After multiple oral administration to male and female rats, triethanolamine was mainly excreted unchanged. The urinary and fecal excretion ratio of unchanged triethanolamine remained constant throughout the treatment period (for five to six days) in both males and females. A small amount of triethanolamine (1.4-2.7%) was excreted as glucuronide conjugates.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V77 390 (2000)
The biotransformation of 14(C)triethanolamine to monoethanolamine and diethanolamine was specifically investigated in mice after both intravenous and dermal treatments. Neither of the hypothetical metabolites was detected in urine (by mass spectral analysis), whereas more than 95% of the radioactivity detected in urine was identified as unchanged triethanolamine. In vitro, triethanolamine had an inhibitory effect on the incorporation of 32(P)phosphate into phospholipids from rabbit and human tissues. Cytochrome P450 monooxygenasedependent oxidative N-dealkylation of triethanolamine does occur in microorganisms, with formation of diethanolamine, ethanolamine and glyoxylate as reaction products.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V77 389 (2000)
To determine potential nitrosation of triethanolamine (TEA) to N-nitrosodiethanolamine (NDELA) at different physiological conditions of the GI tract, in vitro NDELA formation was examined in aqueous reaction mixtures at several pHs (2-10) adjusted with acetic, sulfuric or hydrochloric acids or in cultures of mouse cecal microflora incubated. In vivo NDELA formation was also determined in blood, ingesta, and urine of female B6C3F1 mice after repeated dermal, most relevant human route, or single oral exposure to 1000 mg/kg TEA in the presence of high oral dosages of NaNO(2). Appropriate diethanolamine (DEA) controls were included to account for this impurity in the TEA used. Samples were analyzed for NDELA using GC/MS. The highest degree of nitrosation of TEA to NDELA (approximately 3%) was observed in the in vitro cultures at pH 4 and acetic acid with lower amounts obtained using sulphuric acid ( pproximately 1.3%) and hydrochloric acid (approximately 1.2%). At pH 7, <1% of the TEA was nitrosated to NDELA and at pH 2 (HCl) or pH 10 (NaOH) no NDELA was found above the limit of detection. In incubated cultures containing cecal microflora and nutrient broth, only 0.68% of TEA was nitrosated to NDELA. No NDELA was formed in rats repeatedly dermally dosed with TEA at the limits of detection in blood (0.001 ug/mL, ppm), ingesta (0.006 ug/mL, ppm), and urine (0.47 ug/mL, ppm). Levels of NDELA measured in blood and ingesta after a single oral dose of TEA and NaNO(2) were less than those in DEA controls. These findings in toto confirm the lack of any significant formation of NDELA from TEA in vivo.
PMID:15905009 Saghir SA et al; Regul Toxicol Pharmacol 43 (1): 10-8 (2005)
For more Metabolism/Metabolites (Complete) data for TRIETHANOLAMINE (6 total), please visit the HSDB record page.
The elimination of 14(C)triethanolamine from the blood of mice administered 1.0 mg/kg bw intravenously showed first-order biphasic kinetics with a rapid (0.58-hr half life) and a slow phase (10.2-hr half-life). The slow phase half-lives for elimination of triethanolamine in mice after dermal exposure to 1000 and 2000 mg/kg bw in acetone were 9.7 hr and 18.6 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V77 389 (2000)
The blood half-life of triethanolamine equivalents after iv injection (1 mg/kg) or dermal application (1000 mg/kg) of (14)C-triethanolamine in mice was about 9.5 hr.
Snyder, R. (ed.). Ethel Browning's Toxicity and Metabolism of Industrial Solvents. 2nd ed. Volume II: Nitrogen and Phosphorus Solvents. Amsterdam-New York-Oxford: Elsevier, 1990., p. 443
As an amine, trolamine is capable of accepting a hydrogen to form hydroxide and a conjugate acid. This raises the pH of the solution. As a surfactant, it can lower the interfacial tension in a mixture or solution to prevent separation of emulsions or precipitation of a compound out of solution.