

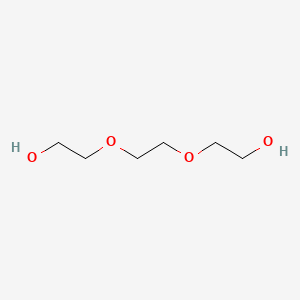

1. 1,2-bis(2-hydroxyethoxy)ethane
2. 2,2'-(1,2-ethanediylbis(oxy))bisethanol
3. 2,2'-(ethylenedioxy)diethanol
4. 2,2'-ethylenedioxybis(ethanol)
5. 3,6-dioxaoctane-1,8-diol
6. Bis(2-hydroxyethoxyethane)
7. Ethylene Glycol Dihydroxydiethyl Ether
8. Polyethylene Glycol (3)
9. Polyethylene Glycol 150
10. Triethylene Glycol, Dipotassium Salt
11. Triethylene Glycol, Disodium Salt
12. Triethylene Glycol, Sodium Salt
1. 112-27-6
2. Triglycol
3. 2,2'-(ethane-1,2-diylbis(oxy))diethanol
4. 2-[2-(2-hydroxyethoxy)ethoxy]ethanol
5. Triethyleneglycol
6. Trigen
7. Triethylenglykol
8. 2,2'-ethylenedioxydiethanol
9. 1,2-bis(2-hydroxyethoxy)ethane
10. 2,2'-(ethylenedioxy)diethanol
11. 2,2'-ethylenedioxybis(ethanol)
12. 3,6-dioxaoctane-1,8-diol
13. Di-beta-hydroxyethoxyethane
14. 2,2'-ethylenedioxyethanol
15. 2,2'-[ethane-1,2-diylbis(oxy)]diethanol
16. Ethanol, 2,2'-[1,2-ethanediylbis(oxy)]bis-
17. Triethylene Glcol
18. Glycol Bis(hydroxyethyl) Ether
19. Ethylene Glycol Dihydroxydiethyl Ether
20. Trigol
21. Teg
22. 2,2'-(1,2-ethanediylbis(oxy))bisethanol
23. Ethanol, 2,2'-(ethylenedioxy)di-
24. Nsc 60758
25. 2-[2-(2-hydroxy-ethoxy)-ethoxy]-ethanol
26. Ethylene Glycol-bis-(2-hydroxyethyl Ether)
27. Di-.beta.-hydroxyethoxyethane
28. 119438-10-7
29. Chebi:44926
30. Ethanol, 2,2'-(1,2-ethanediylbis(oxy))bis-
31. Nsc-60758
32. 3p5su53360
33. Ncgc00163798-03
34. 2-[2-(2-hydroxyethoxy)ethoxy]ethan-1-ol
35. Dsstox_cid_1393
36. Poly(ethylene Glycol) 4-nonylphenyl 3-sulfopropyl Ether, Potassium Salt
37. Dsstox_rid_76135
38. Dsstox_gsid_21393
39. 103734-98-1
40. 122784-99-0
41. 137800-98-7
42. 145112-98-7
43. 2,2'-(ethane-1,2-diylbis(oxy))bis(ethan-1-ol)
44. Caswell No. 888
45. Triethylenglykol [czech]
46. Mfcd00081839
47. Bis(2-hydroxyethoxyethane)
48. Polyethylene Glycol 6,000
49. Cas-112-27-6
50. Hsdb 898
51. Einecs 203-953-2
52. Oh-peg3-oh
53. Epa Pesticide Chemical Code 083501
54. Brn 0969357
55. Trigenos
56. Triethylenglycol
57. Polyethylene Glycol 12,000
58. Polyethylene Glycol 20,000
59. Ai3-01453
60. Ccris 8926
61. Unii-3p5su53360
62. Triethylene-glycol
63. Triethyleneglycol,
64. Tri-ethylene Glycol
65. 3,8-diol
66. Polyethyleneglycol 300
67. Macrogol 150
68. Polyethylene Glycol 1500
69. Ec 203-953-2
70. Polyethylene Glycol 2000
71. Triethylene Glycol, Puriss.
72. Peg-3
73. Polyethylene Glycol 8,000
74. Schembl14929
75. Wln: Q2o2o2q
76. 3,6-dioxa-1,8-octanediol
77. 4-01-00-02400 (beilstein Handbook Reference)
78. Polyethylene Glycol 10,000
79. Amy375
80. Di(2-ethylbutyrate), Diacetate
81. Ethanol,2'-(ethylenedioxy)di-
82. Triethylene Glycol [mi]
83. Chembl1235259
84. Dtxsid4021393
85. Polyethylene Glycol (3)
86. Polyethylene Glycol 150
87. Triethylene Glycol Reagent Grade
88. Triethylene Glycol [hsdb]
89. Triethylene Glycol [inci]
90. Nsc60758
91. Str02345
92. Triethylene Glycol [usp-rs]
93. Triethylene Glycol [who-dd]
94. Zinc1690436
95. Tox21_112073
96. Tox21_202440
97. Tox21_300306
98. Mfcd00002880
99. Mfcd01779596
100. Mfcd01779599
101. Mfcd01779601
102. Mfcd01779603
103. Mfcd01779605
104. Mfcd01779609
105. Mfcd01779611
106. Mfcd01779612
107. Mfcd01779614
108. Mfcd01779615
109. Mfcd01779616
110. Stl282716
111. Akos000120013
112. Triethylene Glycol (industrial Grade)
113. Cs-w018156
114. Db02327
115. Hy-w017440
116. Polyethylene Oxide, M.w. 100,000
117. Polyethylene Oxide, M.w. 300,000
118. Ncgc00163798-01
119. Ncgc00163798-02
120. Ncgc00163798-04
121. Ncgc00163798-05
122. Ncgc00163798-06
123. Ncgc00254097-01
124. Ncgc00259989-01
125. 2-[2-(2-hydroxyethoxy)ethoxy]ethanol #
126. Bp-21036
127. Polyethylene Oxide, M.w. 1,000,000
128. Polyethylene Glycol (peg), 50% Solution
129. Triethylene Glycol, Reagentplus(r), 99%
130. Ethanol,2'-[1,2-ethanediylbis(oxy)]bis-
131. Polyethylene Oxide, M.w. >5,000,000
132. Ft-0652416
133. Ft-0659862
134. T0428
135. F71165
136. Triethylene Glycol, Saj First Grade, >=96.0%
137. Q420630
138. Sr-01000944720
139. Triethylene Glycol, Vetec(tm) Reagent Grade, 98%
140. J-506706
141. Sr-01000944720-1
142. F0001-0256
143. Triethylene Glycol, Bioultra, Anhydrous, >=99.0% (gc)
144. Z1318198494
145. Alpha,omega-bis-hydroxy Poly(ethylene Glycol) (peg-wm 3.000 Dalton)
146. Triethylene Glycol, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 150.17 g/mol |
|---|---|
| Molecular Formula | C6H14O4 |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 150.08920892 g/mol |
| Monoisotopic Mass | 150.08920892 g/mol |
| Topological Polar Surface Area | 58.9 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 49.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Four male albino rats weighing 112 to 145 g were given a single oral dose of 22.5 mg randomly radiolabeled 14-C-triethylene glycol. The rats were then placed in a metabolic chamber in which urine, feces, and expired air were collected over a period of 5 days. The radioactivity recovered (in percent of the administered dose) amounted to 0.8 to 1.2% in expired air, 2.0 to 5.3% in feces, and 86.1 to 94.0% in urine. The total recovery of radioactivity was 90.6% to 98.3% of the administered dose.
PMID:17090481 Cosmetic Ingredient Review Expert Panel; Int J Toxicol 25 (Supp 2): 121-38 (2006)
Following oral dosing, the rat and rabbit excreted most of the triethylene glycol in both unchanged and/or oxidized forms (mono- and dicarboxylic acid derivatives of triethylene glycol). In rabbits dosed with 200 or 2000 mg/kg triethylene glycol respectively excreted 34.3% or 28%, of the administered dose in the urine as unchanged triethylene glycol and 35.2% as a hydroxyacid form of this chemical. In the studies with rats, little if any 14-C-oxalate or 14-C-triethylene glycol in conjugated form was found in the urine. Trace amounts of orally administered 14-C triethylene glycol were excreted in expired air as carbon dioxide (<1%) and in detectable amounts in feces (2 to 5 %). The total elimination of radioactivity (urine, feces and CO2) during the five day period following an oral dose of labeled compound (22.5 mg) ranged from 91 to 98%. The majority of the radioactivity appeared in the urine.
U.S. EPA; TRIETHYLENE GLYCOL - Revised Report of the Antimicrobials Division Toxicology Endpoint Selection Committee. 13 pp. November 21, 2005. Avaliable at https://www.epa.gov.edocket as OPP-2005-0250-0003.
No studies have been reported dealing with the skin absorption of triethylene glycol. Although it is possible that under conditions of very severe prolonged exposures to this chemical, absorption through the skin can occur, it is doubtful any appreciable systemic/dermal injury would occur because triethylene glycol has (1) a low order of dermal irritancy, (2) is not a dermal sensitizer, and (3) showed no evidence of dermal or systemic toxicity following repeated dermal applications of 2 mL (approximately 600 mg/kg) triethylene glycol applied to the skin of rabbits in a 21-day dermal toxicity study.
U.S. EPA; TRIETHYLENE GLYCOL - Revised Report of the Antimicrobials Division Toxicology Endpoint Selection Committee. 13 pp. November 21, 2005. Avaliable at https://www.epa.gov.edocket as OPP-2005-0250-0003.
Two female New Zealand white rabbits triethylene glycol by stomach tube. Urine from the dosed animals was subsequently collected for 24 hrs. Rabbits dosed with 200 or 2,000 mg/kg respectively excreted 34.3% or 28% of the dose amount as unchanged triethylene glycol. The urine of one rabbit contained 35.2% of the administered dose as a hydroxyacid form of triethylene glycol.
PMID:17090481 Cosmetic Ingredient Review Expert Panel; Int J Toxicol 25 (Supp 2): 121-38 (2006)
Triethylene glycol is believed to be metabolized in mammals by alcohol dehydrogenase to acidic products causing metabolic acidosis. Triethylene glycol metabolism by alcohol dehydrogenase can be inhibited by 4-methyl pyrazole or ethanol.
PMID:17090481 Cosmetic Ingredient Review Expert Panel; Int J Toxicol 25 (Supp 2): 121-38 (2006)
... Eliminated ... possibly as mono- and dicarboxylic acid derivatives or triethylene glycol. In studies with rats, little if any 14-C-oxalate or 14-C-triethylene glycol in conjugated form was found in urine.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 3842