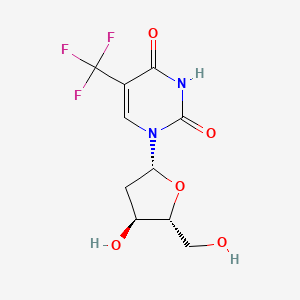

1. 2'-deoxy-5-(trifluoromethyl)uridine
2. 5 Trifluoromethyl 2' Deoxyuridine
3. 5-trifluoromethyl-2'-deoxyuridine
4. Tft Ophtiole
5. Triflumann
6. Trifluoridine
7. Trifluorothymidine
8. Viromidin
9. Virophta
10. Viroptic
1. Trifluorothymidine
2. 70-00-8
3. Viroptic
4. 5-trifluorothymidine
5. Trifluoromethyldeoxyuridine
6. F3dthd
7. Trifluridina
8. Virophta
9. 5-(trifluoromethyl)deoxyuridine
10. F3tdr
11. Trifluridinum
12. 5-trifluoromethyl-2-deoxyuridine
13. Trifluorothymine Deoxyriboside
14. 5-trifluoromethyl-2'-deoxyuridine
15. 1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1h,3h)-dione
16. 2'-deoxy-5-trifluoromethyluridine
17. Tfdu
18. 2'-deoxy-5-(trifluoromethyl)uridine
19. Nsc 75520
20. Nsc 529182
21. Uridine, 2'-deoxy-5-(trifluoromethyl)-
22. Trifluridine (viroptic)
23. Chembl1129
24. Rmw9v5rw38
25. Mls000028361
26. Chebi:75179
27. Nsc-75520
28. 1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(trifluoromethyl)pyrimidine-2,4-dione
29. Nsc-529182
30. F3t
31. Triflurdine (viroptic)
32. Fluridine
33. Smr000058583
34. Trifluridinum [inn-latin]
35. Trifluridina [inn-spanish]
36. Dsstox_cid_26602
37. Dsstox_rid_81757
38. Dsstox_gsid_46602
39. 5-trifluoro-2'-deoxythymidine
40. 5-(trifluoromethyl)-2'-deoxyuridine
41. Alpha,alpha,alpha-trifluorothymidine
42. Cf3durd
43. Viroptic (tn)
44. Thymidine, Alpha,alpha,alpha-trifluoro-
45. Ccris 2348
46. Einecs 200-722-8
47. Mfcd00006534
48. Unii-rmw9v5rw38
49. Brn 0568095
50. Nsc75520
51. Nsc529182
52. Trifluridine [usan:usp:inn]
53. Hsdb 8126
54. Cas-70-00-8
55. Ncgc00166323-01
56. Hs-0007
57. Opera_id_1810
58. 5-trifluoromethylthymidine
59. Trifluridine [mi]
60. Trifluridine [inn]
61. Trifluridine [jan]
62. Trifluoromethyl Deoxyuridine
63. Trifluridine [usan]
64. Cid_6256
65. Schembl3479
66. 2,4(1h,3h)-pyrimidinedione, 1-(2-deoxy-beta-d-ribofuranosyl)-5-(trifluoromethyl)-
67. Trifluridine [vandf]
68. Trifluridine [mart.]
69. Mls001148248
70. Mls006010219
71. Trifluridine [usp-rs]
72. Trifluridine [who-dd]
73. Trifluridine (jan/usp/inn)
74. Gtpl8697
75. Dtxsid4046602
76. Cid_6708818
77. Hms2233n19
78. Hms3715c14
79. Trifluridine [orange Book]
80. Trifluridine [ep Monograph]
81. 5-trifluoromethyl-2''-deoxyuridine
82. Bcp09147
83. Hy-a0061
84. Zinc3842753
85. Tox21_112411
86. Trifluridine [usp Monograph]
87. Bdbm50132298
88. Trifluorothymidine, >=99% (hplc)
89. Akos015919482
90. Tox21_112411_1
91. Ccg-221056
92. Cs-1602
93. Db00432
94. Tas-102 Component Trifluridine
95. Trifluridine Component Of Lonsurf
96. Ncgc00166323-02
97. Ncgc00166323-16
98. S-95005 Component Trifluridine
99. 1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(trifluoromethyl)-1,2,3,4-tetrahydropyrimidine-2,4-dione
100. 1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-(trifluoromethyl)pyrimidine-2,4-dione
101. Sri-10817-12
102. Sri-10817_14
103. Am20100660
104. S1778
105. Sw199522-2
106. T2511
107. D00391
108. Thymidine, .alpha.,.alpha.,.alpha.-trifluoro-
109. 006t534
110. A836733
111. Sr-01000721911
112. J-700255
113. J-700357
114. Q2359590
115. Sr-01000721911-2
116. Brd-k03243820-001-12-1
117. Brd-k03243820-001-25-3
118. Trifluridine, British Pharmacopoeia (bp) Reference Standard
119. Trifluridine;ftd;5-trifluorothymidine;nsc 529182;nsc 75520
120. Trifluridine, United States Pharmacopeia (usp) Reference Standard
121. 1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-(trifluoromethyl)pyrimidine-2,4(1h,3h)-dione
122. 1-((2r,5r)-4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-trifluoromethyl-1h-pyrimidine-2,4-dione
| Molecular Weight | 296.20 g/mol |
|---|---|
| Molecular Formula | C10H11F3N2O5 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 296.06200594 g/mol |
| Monoisotopic Mass | 296.06200594 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 464 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Trifluridine |
| PubMed Health | Trifluridine (Into the eye) |
| Drug Classes | Antiviral |
| Drug Label | Trifluridine Ophthalmic Solution (also known as trifluorothymidine, F3TdR, F3T), an antiviral drug for topical treatment of epithelial keratitis caused by herpes simplex virus. The chemical name of trifluridine is ,,-trifluorothymidine. Triflur... |
| Active Ingredient | Trifluridine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Viroptic |
| PubMed Health | Trifluridine (Into the eye) |
| Drug Classes | Antiviral |
| Drug Label | VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine, F3TdR,F3T), an antiviral drug for topical treatment of epithelial keratitis caused by herpes simplex virus. The chemical name of trifluridine is ,, -trifluorothymidi... |
| Active Ingredient | Trifluridine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Monarch Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Trifluridine |
| PubMed Health | Trifluridine (Into the eye) |
| Drug Classes | Antiviral |
| Drug Label | Trifluridine Ophthalmic Solution (also known as trifluorothymidine, F3TdR, F3T), an antiviral drug for topical treatment of epithelial keratitis caused by herpes simplex virus. The chemical name of trifluridine is ,,-trifluorothymidine. Triflur... |
| Active Ingredient | Trifluridine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Viroptic |
| PubMed Health | Trifluridine (Into the eye) |
| Drug Classes | Antiviral |
| Drug Label | VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine, F3TdR,F3T), an antiviral drug for topical treatment of epithelial keratitis caused by herpes simplex virus. The chemical name of trifluridine is ,, -trifluorothymidi... |
| Active Ingredient | Trifluridine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Monarch Pharms |
Trifluridine Ophthalmic Solution is indicated for the treatment of primary keratoconjunctivitis and recurrent epithelial keratitis due to herpes simplex virus, types 1 and 2. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for TRIFLURIDINE solution (August 2011). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=abc1d07f-91ee-4421-bc9b-457d0ed41f4c
Trifluridine is also effective in the treatment of epithelial keratitis that has not responded clinically to the topical administration of idoxuridine or when ocular toxicity or hypersensitivity to idoxuridine has occurred. In a smaller number of patients found to be resistant to topical vidarabine, trifluridine was also effective.
US Natl Inst Health; DailyMed. Current Medication Information for TRIFLURIDINE solution (August 2011). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=abc1d07f-91ee-4421-bc9b-457d0ed41f4c
Trifluridine 1% ophthalmic solution is contraindicated in patients who have chemical intolerance or are hypersensitive to the drug or any ingredient in the formulation.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Trifluridine should be used only under the close supervision of an ophthalmologist. In addition, the recommended dosage and frequency of administration should not be exceeded.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
If trifluridine is used in smallpox vaccinees who have vaccinia lesions on or near the eyelid for prophylaxis to prevent extension of vaccinia infection to the conjunctiva or cornea, the potential benefit of the drug should be balanced against the minimal but potential risk of drug toxicity and of introducing the virus into the eye by frequent manipulation.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
US Natl Inst Health; DailyMed. Current Medication Information for TRIFLURIDINE solution (August 2011). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=abc1d07f-91ee-4421-bc9b-457d0ed41f4c
For more Drug Warnings (Complete) data for Trifluridine (8 total), please visit the HSDB record page.
Trifluridine is used for the treatment of primay keratoconjunctivitis and recurrent epithelial keratitis due to herpes simplex virus, types 1 and 2 in ophthalmic solutions. Trifluridine, in combination with tipiracil as oral tablets, is indicated for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy.
FDA Label
Trifluridine exhibits an antiviral effect against herpes simplex virus, types 1 and 2 and vacciniavirus both in vitro and in vivo. Some strains of adenovirus that contribute to the pathology of keratoconjunctivitis were shown to be susceptible to trifluridine _in vitro_. While there is evidence from a study that cross-resistance may develop between trifluridine and [idoxuridine] or [vidarabine], trifluridine was shown to effective in treating dendritic ulcers in patients with herpetic keratitis who are unresponsive to [idoxuridine] or [vidarabine] based on the results from masked comparative trials. In nonclinical studies, trifluridine/tipiracil hydrochloride demonstrated antitumour activity against both 5-fluorouracil (5-FU) sensitive and resistant colorectal cancer cell lines. The cytotoxic activity of trifluridine and tipiracil against several human tumour xenografts show high correlation with the amount of trifluridine incorporated into DNA, indicating that the primary mechanism of action of trifluridine involves the direct incorporation into the cancer cell DNA. Trifluridine and tipiracil demonstrated anti-tumor activity against KRAS wild-type and mutant human colorectal cancer xenografts in mice. In clinical studies comprised of patients with previously treated metastatic colorectal cancer, treatment of trifluridine in combination with tipiracil in addition to best supportive care over a 5- or 7-month period resulted in increased progression-free survival (PFS), overall response rate (ORR) and disease control rate (DCR) compared to placebo. In an open-label study, administration of trifluridine at the recommended dosage in patients with advanced solid tumors had no clinically relevant effect on QT/QTc prolongation compared with placebo. Two out of 48 patients displayed had QTc greater than 500 msec and 1 of 42 patients (2.4%) had a QTc increase from baseline greater than 60 msec.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
L01BC59
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AD - Antivirals
S01AD02 - Trifluridine
Absorption
Systemic absorption of trifluridine following therapeutic dosing with ophthalmic trifluridine appears to be negligible. At least 57% of the orally-administered trifluridine is absorbed. Following twice daily oral dosing of trifluridine in combination with tipiracil, systemic exposure of trifluridine increased more than dose-proportionally over the dose range of 15 to 35 mg/m^2. Trifluridine accumulation was 3-fold for AUC0-last and 2-fold for peak plasma concentration (Cmax) at steady state. The time to reach the peak plasma concentrations (Cmax) was 2 hours. Tipiracil increases the AUC and Cmax of trifluridine. Food intake was shown to decrease the Cmax and AUC compared to those in a fasting state.
Route of Elimination
About 55% of the total recovered radio-labelled trifluridine was detected in the urine within 24 hours. Following oral administration of trifluridine in combination with tipiracil, only 3% of the total dose was excreted into faeces and expired air. Administration of a 60mg single oral dose of the combination product resulted in the mean urinary recovery of 1.5% of unchanged trifluridine and 19.2% for the inactive metabolite FTY after the 48-hour cumulative excretion.
Volume of Distribution
Following a single dose of Lonsurf (35 mg/m2) in patients with advanced solid tumours, the apparent volume of distribution (Vd/F) for trifluridine was 21 L.
Clearance
Following a single dose of Lonsurf (35 mg/m^2) in patients with advanced solid tumours, the oral clearance (CL/F) for trifluridine was 10.5 L/hr.
Following topical application of trifluridine to the eye, the drug penetrates the cornea and can be detected in the aqueous humor.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Systemic absorption following ocular application of trifluridine appears to be negligible. In one study in healthy individuals, topical application of trifluridine 1% ophthalmic solution to the eyes 7 times daily for 14 consecutive days did not result in detectable serum concentrations of trifluridine or 5-carboxy-2'-deoxyuridine.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
During in vitro studies using excised rabbit corneas, the major metabolite of trifluridine, 5-carboxy-2'-deoxyuridine, was found on the endothelial side of the cornea in addition to the parent compound; however, detectable levels of the metabolite have not been found in the aqueous humor in humans.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
It is unlikely that trifluridine is excreted in human milk after ophthalmic instillation of trifluridine because of the relatively small dosage (
US Natl Inst Health; DailyMed. Current Medication Information for TRIFLURIDINE solution (August 2011). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=abc1d07f-91ee-4421-bc9b-457d0ed41f4c
One major metabolite, 5-carboxy-2'-deoxyuridine found on the endothelial side of the cornea, indicating localized metabolism. Trifluridine is mainly eliminated by metabolism via thymidine phosphorylase (TPase) to form an inactive metabolite, 5-(trifluoromethyl) uracil (FTY).
The major metabolite of trifluridine (5-carboxy-2'-deoxyuridine appears) to have some antiviral activity but substantially less than that of the parent drug.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
19F NMR spectroscopy has been used to study further the metabolism of 5-trifluoromethyl-2'-deoxyuridine (trifluridine; F3TdR). The synthesis and characterization of alpha-trifluoromethyl-beta-alanyl glycine (F3MBAG), a putative new metabolite of F3TdR, are now reported. This study describes ex vivo and in vivo detection of F3MBAG and other previously reported metabolites of trifluridine, using 19F NMR spectroscopy, in male BALB/C mice bearing EMT-6 tumors. A parallel 19F NMR spectroscopic study was also performed on rats dosed with F3TdR, to observe the qualitative pattern of F3TdR metabolism in another species. Unexpectedly, 5-trifluoromethyl-5,6-dihydroxyuracil (DOHF3T), alpha-trifluoromethyl-beta-ureidopropionic acid (F3MUPA) and fluoride, which result from the metabolic degradation of F3TdR and which were detected in various biological samples from mice dosed with F3TdR, could not be identified in rat urine or in homogenized tissue extracts. The presence of these metabolites in intact tissues is uncertain since in this study 19F NMR spectroscopy of these samples always displayed a broad resonance "hump" across the range of chemical shifts that would encompass these metabolites. No clear explanation for the loss of spectroscopic resolution in this region has been rationalized. N-Carboxy-alpha-trifluoromethyl-beta-alanine (F3MBA-CO2), alpha-trifluoromethyl-beta-alanyl alanine (F3MBAA) and N-acetyl-alpha-trifluoromethyl-beta-alanine (Ac-F3MBA) were synthesized and characterized, but were not detected as metabolites in any of the biological specimens examined.
PMID:8093091 Tandon M et al; Biochem Pharmacol. 48 (5):1033-41 (1994)
The half life is 12 to 18 minutes following ophthalmic administration. After administration of trifluridine with tipiracil at oral doses 35 mg/m^2 twice daily, the mean elimination half-life (t1/2) of trifluridine was 1.4 hours following a single dose. At steady state, the mean elimination half-life was 2.1 hours.
The mechanism of action of trifluridine as an antiviral agent has not been fully elucidated, but appears to involve the inhibition of viral replication. Trifluridine gets incorporated into viral DNA during replication, which leads to the formation of defective proteins and an increased mutation rate. Trifluridine also mediates antineoplastic activities via this mechanism; following uptake into cancer cells, trifluridine is rapidly phosphorylated by thymidine kinase to its active monophosphate form. Subsequent phosphorylation produces trifluridine triphosphate, which is readily incorporated into the DNA of tumour cells in place of thymidine bases to perturb DNA function, DNA synthesis, and tumour cell proliferation. As trifluridine is subject to rapid degradation by TPase and readily metabolised by a first-pass effect following oral administration, tipiracil is added in the antineoplastic combination product as an inhibitor of TPase to increase the bioavailability of trifluridine. Trifluridine monophosphate also reversibly inhibits thymidylate synthetase (TS), an enzyme that is necessary for DNA synthesis and which levels are shown to be elevated different cancer cell lines. Up-regulation of the expression of the TS enzyme may also lead to the resistance to antineoplastic therapies, such as 5-fluorouracil (5-FU). However, this inhibitory effect is not considered to be sufficient enough to fully contribute to the cytotoxicity in cancer cells.
Trifluridine is a fluorinated pyrimidine nucleoside with in vitro and in vivo activity against herpes simplex virus, types 1 and 2 and vacciniavirus. Some strains of adenovirus are also inhibited in vitro. ...Trifluridine interferes with DNA synthesis in cultured mammalian cells. However, its antiviral mechanism of action is not completely known
US Natl Inst Health; DailyMed. Current Medication Information for TRIFLURIDINE solution (August 2011). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=abc1d07f-91ee-4421-bc9b-457d0ed41f4c
The exact mechanism of antiviral activity of trifluridine has not been fully elucidated, but appears to involve inhibition of viral replication. Trifluridine, instead of thymidine, is incorporated into viral DNA during replication which results in the formation of defective proteins and an increased mutation rate. Trifluridine also reversibly inhibits thymidylate synthetase, an enzyme required for DNA synthesis.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Trifluridine has shown antiviral activity in vitro and in vivo against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). The drug is active in vitro against vaccinia virus and has shown in vivo activity in the treatment of vaccinia keratitis in rabbits. Trifluridine also has shown antiviral activity in cell culture against some strains of adenovirus. Trifluridine is inactive against bacteria, fungi, and Chlamydia.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012