
1. Trigonelline Chloride
2. Trigonelline Iodide
3. Trigonelline Ion
4. Trigonelline Tosylate
1. 535-83-1
2. Trigenolline
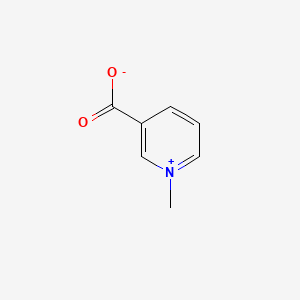
3. N-methylnicotinate
4. Caffearine
5. Gynesine
6. Coffearine
7. Betain Nicotinate
8. 1-methylpyridinium-3-carboxylate
9. Trigonellin
10. Coffearin
11. Betaine Nicotinate
12. 1-methylpyridinio-3-carboxylate
13. Nicotinic Acid N-methylbetaine
14. 1-methylpyridin-1-ium-3-carboxylate
15. N-methylnicotinic Acid
16. Trigenelline
17. Caffearin
18. 1-methyl-3-pyridiniumcarboxylate
19. 3-carboxy-1-methylpyridinium Hydroxide Inner Salt
20. N'-methylnicotinate
21. Chebi:18123
22. 3nq9n60i00
23. Pyridinium, 3-carboxy-1-methyl-, Hydroxide, Inner Salt
24. 1-methyl-nicotinic Acid Anion(trigonelline)
25. 3-carboxy-1-methylpyridinium Hydroxide, Inner Salt
26. 1-methylpyridin-1-ium-3-carboxylic Acid
27. Ccris 1332
28. Einecs 208-620-5
29. Brn 3905114
30. Unii-3nq9n60i00
31. Hsdb 7684
32. 1-methylnicotinate
33. Trigonelline,(s)
34. N-methyl-nicotinate
35. 3-carboxy-1-methylpyridinium Inner Salt
36. N'-methylnicotinic Acid
37. 6138-40-5
38. Trigonelline [mi]
39. Trigonelline [hsdb]
40. 5-22-02-00143 (beilstein Handbook Reference)
41. Mls002153895
42. N-methylnicotinic Acid Betaine
43. Schembl195666
44. Spectrum1500880
45. Trigonelline [usp-rs]
46. Chembl350675
47. Dtxsid2026230
48. Hms2096h08
49. Hms2234k22
50. Hms3371k21
51. Hy-n0414
52. Bdbm50548713
53. Ccg-38517
54. Mfcd00054262
55. Nsc714350
56. Akos005067859
57. Nsc-714350
58. Sdccgmls-0066739.p001
59. Ncgc00095649-01
60. Ncgc00095649-02
61. Ncgc00095649-03
62. Ncgc00095649-04
63. Ncgc00095649-07
64. Ac-34290
65. As-17722
66. Smr001233244
67. Ab00052974
68. Cs-0008944
69. Ft-0689338
70. N2788
71. 1-methyl-5-(oxylatocarbonyl)pyridinium-3-ide
72. Pyridinium, 3-carboxy-1-methyl-, Inner Salt
73. A14825
74. C01004
75. 3-carboxy-1-methyl-pyridinium Hydroxide Inner Salt
76. Q928965
77. 3-carboxy-1-methylpyridinium, Hydroxide, Inner Salt
78. W-105723
79. Trigonelline (constituent Of Fenugreek Seed) [dsc]
| Molecular Weight | 137.14 g/mol |
|---|---|
| Molecular Formula | C7H7NO2 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 137.047678466 g/mol |
| Monoisotopic Mass | 137.047678466 g/mol |
| Topological Polar Surface Area | 44 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 130 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Fenugreek seeds are known for their characteristic smell of soup seasoning and as an ingredient of Indian curry. Traditionally the seeds are used as macerate for the treatment of diabetes, cough, and flatulence, to increase breast milk secretion, and for anti-inflammatory and aphrodisiac effects. The use is limited by its unpleasant smell and bitter taste which can be modified by adding mint leaves to the macerate. Antidiabetic properties are attributed mainly to galactomannan, 4-hydroxyisoleucin (4-OH-Ile), diosgenin and trigonelline. These substances demonstrate direct antidiabetic properties in clinical studies by increasing insulin secretion (4-OH-Ile), decreasing insulin resistance and glucose resorption from the GIT (galactomannan) and improvement in B-cells regeneration (trigonelline). Besides this main effect, the herb improves blood lipid spectre (4-OH-Ile, diosgenin), and has reno-protective (4-OH-Ile, trigonelline), neuroprotective (trigonelline) and antioxidant (diosgenin, trigonelline) effects. Antidiabetic efficacy of trigonelline is comparable to glibenclamide treatment and more effective than sitagliptine therapy. Given the large body of evidence and promising results in comparison with standard pharmacotherapy, fenugreek active substances have a potential to become a source of new antidiabetic medication.Key words: fenugreek Trigonella foenum-graecum diabetes mellitus type 2 biological activity.
PMID:26400229 Koupy D et al; Ceska Slov Farm 64 (3): 67-71 (2015)
/EXPL THER/ There is evidence that Trigonella foenum-graecum L. (fenugreek), a traditional Chinese herb, and its components are beneficial in the prevention and treatment of diabetes and central nervous system disease. The pharmacological activities of trigonelline, a major alkaloid component of fenugreek, have been more thoroughly evaluated than fenugreek's other components, especially with regard to diabetes and central nervous system disease. Trigonelline has hypoglycemic, hypolipidemic, neuroprotective, antimigraine, sedative, memory-improving, antibacterial, antiviral, and anti-tumor activities, and it has been shown to reduce diabetic auditory neuropathy and platelet aggregation. It acts by affecting beta cell regeneration, insulin secretion, activities of enzymes related to glucose metabolism, reactive oxygen species, axonal extension, and neuron excitability. However, further study of trigonelline's pharmacological activities and exact mechanism is warranted, along with application of this knowledge to its clinical usage. This review aims to give readers a survey of the pharmacological effects of trigonelline, especially in diabetes, diabetic complications and central nervous system disease. In addition, because of its pharmacological value and low toxicity, the reported adverse effects of trigonelline in experimental animal models and humans are briefly reviewed, and the pharmacokinetics of trigonelline are also discussed.
PMID:22680628 Zhou J et al; Curr Med Chem. 2012;19(21):3523-31 (2012)
... The concentration-time curves of trigonelline in rabbits after ... iv administration were shown to fit one-compartment and two-compartment open model, respectively. The main parameters after iv /administration/ of trigonelline were as follows: T1/2 alpha was 10.8 min, T1/2 beta was 44.0 min, K21 was 0.044 min-1, K10 was 0.026 min-1, K12 was 0.017 min-1, AUC was 931.0 mg.min/L . /It was concluded that/ trigonelline showed a middle rate of absorption and fast rate of elimination in rabbit...
PMID:12889128 Zhao HQ et al; Yao Xue Xue Bao 38 (4): 279-82 (2003)
... Trigonelline (N-methylnicotinic acid) /is a metabolite of nicotinamide/.
PMID:8082753 Berglund T; FEBS Lett 351 (2): 145-9 (1994)