

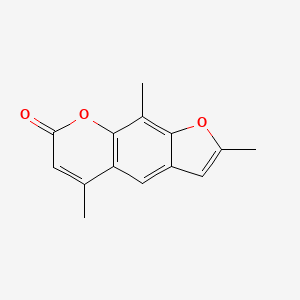

1. 2,5,9-trimethyl-7h-furo(3,2-g)benzopyran-7-one
2. 4,5',8-trimethylpsoralen
3. Nsc 71047
4. Nsc-71047
5. Nsc71047
6. Trimethylpsoralen
7. Trioxisalenum
8. Trioxysalen
9. Trisoralen
1. 3902-71-4
2. Trioxysalen
3. Trisoralen
4. Trimethylpsoralen
5. 4,5',8-trimethylpsoralen
6. 2',4,8-trimethylpsoralen
7. Nsc-71047
8. 2,5,9-trimethyl-7h-furo[3,2-g]chromen-7-one
9. Elder 8011
10. Trioxisaleno
11. Trioxysalene
12. Trioxysalenum
13. 4,5',8-trimethylpsoralene
14. Nsc71047
15. 2,5,9-trimethylfuro[3,2-g]chromen-7-one
16. 7h-furo[3,2-g][1]benzopyran-7-one, 2,5,9-trimethyl-
17. Tmp (psoralen)
18. 4,8,5'-trimethylpsoralen
19. 2,5,9-trimethylfuro[3,2-g]benzopyran-7-one
20. Trioxysalen [inn]
21. Trioxsalin
22. 2,5,9-trimethyl-7h-furo(3,2-g)(1)benzopyran-7-one
23. Mfcd00005010
24. Trisoralen;trioxysalen;tmp
25. Y6uy8ov51t
26. 7h-furo(3,2-g)(1)benzopyran-7-one, 2,5,9-trimethyl-
27. Chebi:28329
28. Ncgc00016643-01
29. Cas-3902-71-4
30. Dsstox_cid_3716
31. Dsstox_rid_77160
32. Dsstox_gsid_23716
33. Trioxsalen [usan]
34. 6-hydroxy-beta,2,7-trimethyl-5-benzofuranacrylic Acid, Delta-lactone
35. Trioxysalene [inn-french]
36. Trioxysalenum [inn-latin]
37. Trioxisaleno [inn-spanish]
38. Nsc 71047
39. Lactone
40. Sr-01000812969
41. Trioxsalen [usan:usp]
42. Einecs 223-459-0
43. 2,5,9-trimethylpsoralen
44. 4,5,8-trimethylpsoralen
45. Unii-y6uy8ov51t
46. Antipsoriatic
47. Brn 0221723
48. Trioxalen
49. Trixsalen
50. Trisoralen (tn)
51. Trioxsalen (usp)
52. Prestwick_148
53. 7-oxo-2,5,9-trimethyl-7h-furo(3,2-g)(1)benzopyron
54. 4,8-trimethylpsoralen
55. Spectrum_000169
56. 4,8-trimethylpsoralene
57. Trioxsalen [mi]
58. 2',8-trimethylpsoralen
59. Trioxysalen (jan/inn)
60. 6-hydroxy-beta,2,7-trimethyl-5-benzofuranacrylic Acid Gamma-lactone
61. Prestwick0_000709
62. Prestwick1_000709
63. Prestwick2_000709
64. Prestwick3_000709
65. Spectrum2_001083
66. Spectrum3_001378
67. Spectrum4_000883
68. Spectrum5_001557
69. Trioxysalen [jan]
70. Trioxsalen [vandf]
71. Schembl1252
72. Chembl1475
73. Trioxsalen [usp-rs]
74. Trioxysalen [mart.]
75. Oprea1_203343
76. Bspbio_000897
77. Bspbio_002936
78. Kbiogr_001286
79. Kbioss_000649
80. Trioxysalen [who-dd]
81. 5-19-04-00472 (beilstein Handbook Reference)
82. Mls001173417
83. Divk1c_000380
84. Spectrum1500596
85. Spbio_001126
86. Spbio_002818
87. Bpbio1_000987
88. Megxm0_000462
89. Zinc2226
90. Dtxsid3023716
91. Trioxsalen [orange Book]
92. Acon0_000603
93. Acon1_000251
94. Hms501c22
95. Kbio1_000380
96. Kbio2_000649
97. Kbio2_003217
98. Kbio2_005785
99. Kbio3_002156
100. Trioxsalen [usp Impurity]
101. Ninds_000380
102. Hms1570m19
103. Hms1921i05
104. Hms2092a12
105. Hms2097m19
106. Hms2879m06
107. Hms3714m19
108. Pharmakon1600-01500596
109. 7h-furo[3, 2,5,9-trimethyl-
110. Hy-b1157
111. Tox21_110541
112. Ccg-39937
113. Nsc757371
114. Stl564342
115. Akos015912601
116. Tox21_110541_1
117. Trioxsalen, >=98% (hplc), Powder
118. Cs-4760
119. Db04571
120. Ks-5184
121. Nsc-757371
122. Sdccgmls-0066609.p001
123. Trioxysalen 100 Microg/ml In Methanol
124. Idi1_000380
125. Ncgc00016643-02
126. Ncgc00016643-03
127. Ncgc00016643-04
128. Ncgc00016643-05
129. Ncgc00016643-06
130. Ncgc00016643-09
131. Ncgc00016643-10
132. Ncgc00094804-01
133. Ncgc00094804-02
134. Ncgc00178384-01
135. Ncgc00178384-02
136. Ncgc00178384-03
137. Nci60_038883
138. Smr000538905
139. 4,5',8-trimethylpsoralen [iarc]
140. Sbi-0051547.p002
141. Db-049366
142. Ft-0603651
143. T2267
144. Benzofuranacrylic Acid,2,7-trimethyl-,
145. C09314
146. D01034
147. T72416
148. 4,7,9-trimethyl-2h-furo[3,2-g]chromen-2-one
149. Ab00052119_09
150. 2,5,9-trimethyl-7-furo[3,2-g][1]benzopyranone
151. 902t714
152. A824380
153. Q854263
154. 2,5,9-trimethyl-7h-furo[3,2-g]chromen-7-one #
155. Sr-01000812969-2
156. Sr-01000812969-5
157. Sr-01000812969-6
158. Sr-01000812969-7
159. 2,9-trimethyl-7h-furo[3,2-g][1]benzopyran-7-one
160. Brd-k54790157-001-06-4
161. Brd-k54790157-001-10-6
162. Z1551429738
163. 5-benzofuranacrylic Acid,2,7-trimethyl-, .delta.-lactone
164. Trioxsalen, United States Pharmacopeia (usp) Reference Standard
165. 5-benzofuranacrylic Acid, 6-hydroxy-.beta.,2,7-trimethyl-, .delta.-lactone
| Molecular Weight | 228.24 g/mol |
|---|---|
| Molecular Formula | C14H12O3 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 228.078644241 g/mol |
| Monoisotopic Mass | 228.078644241 g/mol |
| Topological Polar Surface Area | 39.4 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 374 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Trioxsalen is a pigmenting photosensitizing agent used in conjunction with ultraviolet light in the treatment of vitiligo.
Trioxsalen ispharmacologically inactive but when exposed to ultraviolet radiation or sunlight it is converted to its active metabolite to produce a beneficial reaction affecting the diseased tissue.
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
D - Dermatologicals
D05 - Antipsoriatics
D05A - Antipsoriatics for topical use
D05AD - Psoralens for topical use
D05AD01 - Trioxysalen
D - Dermatologicals
D05 - Antipsoriatics
D05B - Antipsoriatics for systemic use
D05BA - Psoralens for systemic use
D05BA01 - Trioxysalen
After photoactivation it creates interstrand cross-links in DNA, which can cause programmed cell death.
