

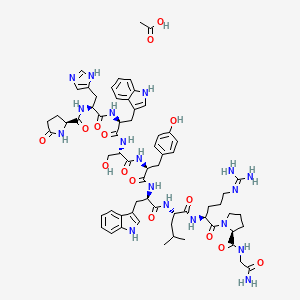

1. 6-d-tryptophan-luteinizing Hormone-releasing Factor (pig)
2. Ay 25650
3. Ay-25650
4. Ay25650
5. Cl 118532
6. Cl-118532
7. Cl118532
8. D-trp-6-lh-rh
9. Decapeptyl
10. Decapeptyl Depot
11. Decapeptyl Lp
12. Decapeptyl Trimestral
13. Embonate, Triptorelin
14. Gnrh, Trp(6)-
15. Lhrh, Trp(6)-
16. Lhrh, Tryptophyl(6)-
17. Pamoate, Triptorelin
18. Trelstar
19. Trimestral, Decapeptyl
20. Triptorelin
21. Triptorelin Embonate
22. Triptorelin Pamoate
23. Wy 42462
24. Wy-42462
25. Wy42462
1. 140194-24-7
2. 140194-24-7 (acetate)
3. Bim 21003
4. Wy 42422
5. Wy 42462
6. Ay 25650
7. Pglu-his-trp-ser-tyr-d-trp-leu-arg-pro-gly-nh2 Acetate
8. Decapeptyl (tn)
9. (s)-n-(2-amino-2-oxoethyl)-1-(((s)-5-oxopyrrolidine-2-carbonyl)-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-tryptophyl-l-leucyl-l-arginyl)pyrrolidine-2-carboxamide Acetate
10. Unii-43ofw291r9
11. Bim 21003c
12. 43ofw291r9
13. Dtxsid301033427
14. 6-d-tryptophan Luteinizing Hormone-releasing Factor (swine) Acetate
15. Hy-12551a
16. Akos030485974
17. Cs-0012388
18. Propan-2-yl]-5-oxopyrrolidine-2-carboxamide
19. D08649
20. J-007369
21. Q27258659
22. Luteinizing Hormone-releasing Factor (swine), 6-d-tryptophan-, Acetate (1:1)
23. 2-yl]amino]-3-(1h-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-(1h-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(1h-imidazol-4-yl)-1-oxo
24. Acetic Acid;(2s)-n-[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2r)-1-[[(2s)-1-[[(2s)-1-[(2s)-2-[(2-amino-2-oxoethyl)carbamoyl]pyrrolidin-1-yl]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-
| Molecular Weight | 1371.5 g/mol |
|---|---|
| Molecular Formula | C66H86N18O15 |
| Hydrogen Bond Donor Count | 18 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 33 |
| Exact Mass | 1370.65200411 g/mol |
| Monoisotopic Mass | 1370.65200411 g/mol |
| Topological Polar Surface Area | 528 Ų |
| Heavy Atom Count | 99 |
| Formal Charge | 0 |
| Complexity | 2750 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
For the synchronisation of ovulation in weaned sows to enable a single fixed-time artificial insemination.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Luteolytic Agents
Chemical compounds that cause LUTEOLYSIS or degeneration of the CORPUS LUTEUM. (See all compounds classified as Luteolytic Agents.)
QH01CA97
