


1. Tegunor
2. Tricosal
3. Trilisate
1. 64425-90-7
2. Djj95fjp1h
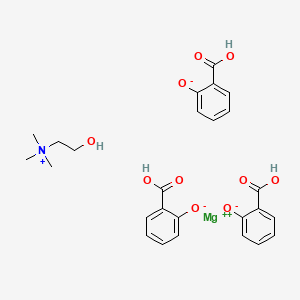
3. Choline Magnesium Salicylate
4. Cholinemagnesiumtrisalicylate
5. Trisalicylate-choline
6. Tegunor
7. Tricolsal
8. Unii-djj95fjp1h
9. Tricosal (tn)
10. Choline Mixture With Magnesium Salicylate
11. Dtxsid901344133
12. Db01401
13. Nsc 751675
14. D07694
15. Choline Magnesium Trisalicylate [who-dd]
16. Q20817211
17. ((1,2-ethanediylbis(carbamodithioato))(2-))manganese, Mixt. With ((1,2-ethanediylbis(carbamodithioato))(2-))zinc & 2(or 4)-isooctyl-4,6(or 2,6)-dinitrophenyl 2-buten Oate
18. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-, 2-hydroxybenzoate (1:1), Mixt. With (t-4)-bis(2-(hydroxy-.kappa.o)benzoato-.kappa.o)magnesium
19. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-, Salt With 2-hydroxybenzoic Acid (1:1), Mixt. With 2-hydroxybenzoic Acid Magnesium Salt (2:1)
| Molecular Weight | 539.8 g/mol |
|---|---|
| Molecular Formula | C26H29MgNO10 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 539.1641878 g/mol |
| Monoisotopic Mass | 539.1641878 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 179 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
Choline magnesium trisalicylate is used to reduce pain and inflammation caused by conditions such as arthritis. This medication is also used to treat fever in adults.
Trisalicylate-choline is a non-steroidal anti-inflammatory drug (NSAID) that contains a combination of choline salicylate and magnesium salicylate. Does not affect platelet aggregation.
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Route of Elimination
renal
Inhibits prostaglandin synthesis; acts on the hypothalamus heat-regulating center to reduce fever; blocks the generation of pain impulses