

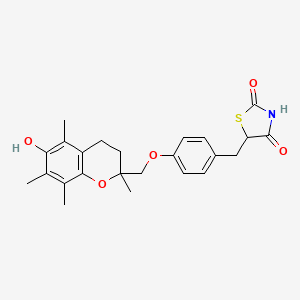

1. 5-(4-((6-hydroxy-2,5,7,8-tetramethylchroman-2-yl-methoxy)benzyl)-2,4-thiazolidinedione) - T
2. Cs 045
3. Cs-045
4. Cs045
5. Prelay
6. Rezulin
1. 97322-87-7
2. Rezulin
3. Romglizone
4. Prelay
5. Cs-045
6. Romozin
7. Noscal
8. Rezulin (tn)
9. Cs 045
10. Gr 92132x
11. Ccris 8969
12. Gr92132x
13. Ci 991
14. Ci-991
15. Gr-92132x
16. Chebi:9753
17. (+-)-all-rac-5-(p-((6-hydroxy-2,5,7,8-tetramethyl-2-chromanyl)methoxy)benzyl)-2,4-thiazolidinedione
18. 5-(4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl)thiazolidine-2,4-dione
19. Troglitazone (cs-045)
20. (+/-)-5-[4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]benzyl]-2,4-thiazolidinedione
21. 5-(4-((6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy)benzyl)thiazolidine-2,4-dione
22. 5-[[4-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydrochromen-2-yl)methoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
23. Ncgc00164445-01
24. Dsstox_cid_3719
25. Dsstox_rid_77162
26. Dsstox_gsid_23719
27. 2,4-thiazolidinedione, 5-((4-((3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2h-1-benzopyran-2-yl)methoxy)phenyl)methyl)-
28. 5-{4-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2h-chromen-2-yl)methoxy]benzyl}-1,3-thiazolidine-2,4-dione
29. Troglitazona
30. Troglitazonum
31. Bdbm50101974
32. 5-[(4-{[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2h-chromen-2-yl)methyl]oxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione
33. Cas-97322-87-7
34. Sr-05000000454
35. 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2h-1-benzopyran-2-yl)methoxy]phenyl]methyl]-2,4-thiazolidinedione
36. Unii-i66zz0zn0e
37. Brn 4338399
38. Troglitazone (jan/usan/inn)
39. Troglitazone [usan:inn:ban]
40. 5-[4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl]thiazolidine-2,4-dione
41. Cs045
42. 2,4-thiazolidinedione, 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2h-1-benzopyran-2-yl)methoxy]phenyl]methyl]-
43. Spectrum5_001973
44. Upcmld-dp017
45. Schembl4959
46. I66zz0zn0e
47. Mls006010817
48. Gtpl2693
49. Chembl3542292
50. Dtxsid8023719
51. Upcmld-dp017:001
52. Upcmld-dp017:002
53. Troglitazone, >=98% (hplc)
54. Hms2089d22
55. Hms2093d04
56. Hms3649g12
57. Hms3713d08
58. Bcp06753
59. Ex-a3782
60. Tox21_112119
61. Tox21_300470
62. Bdbm50088494
63. Hsci1_000037
64. S8432
65. Akos000281116
66. Akos024457434
67. Tox21_112119_1
68. Troglitazone - Cas 97322-87-7
69. Ccg-208125
70. Cs-1634
71. Db00197
72. Smp2_000224
73. Ncgc00161599-01
74. Ncgc00161599-02
75. Ncgc00161599-03
76. Ncgc00161599-04
77. Ncgc00161599-05
78. Ncgc00161599-06
79. Ncgc00161599-07
80. Ncgc00161599-08
81. Ncgc00161599-09
82. Ncgc00161599-11
83. Ncgc00254440-01
84. Ac-31453
85. As-56378
86. Hy-50935
87. Smr001550129
88. Db-057670
89. Ft-0630994
90. T3920
91. D00395
92. H12073
93. Ab00643330-02
94. 322t877
95. A845704
96. Q7844989
97. Sr-05000000454-2
98. Sr-05000000454-3
99. Sr-05000000454-5
100. Brd-a13084692-001-02-5
101. 5-[4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl]-2,4-dioxothiazolidine
102. 5-[4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl]thiazolidine -2,4-dione
103. (5r)-5-[[4-[[(2s)-6-hydroxy-2,5,7,8-tetramethyl-chroman-2-yl]methoxy]phenyl]methyl]thiazolidine-2,4-dione
104. 2,4-thiazolidinedione, 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2h-1-benzopyran-2-yl)methoxy]phenyl]methyl]- (9ci)
105. 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2h-1- Benzopyran-2-yl)methoxy]phenyl]methyl]-2,4-thiazlidinedione
106. 5-[[4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
107. 5-[4-(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2h-1-benzopyran-2-ylmethoxy)benzyl]thiazolidine-2,4-dione
108. 5-{4-(6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-yl-methoxy) Benzyl) Thiazolidine-2,4-dione
| Molecular Weight | 441.5 g/mol |
|---|---|
| Molecular Formula | C24H27NO5S |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 441.16099414 g/mol |
| Monoisotopic Mass | 441.16099414 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 681 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of Type II diabetes mellitus. It is used alone or in combination with a sulfonylurea, metformin, or insulin as an adjunct to diet and exercise.
FDA Label
Troglitazone is an oral antihyperglycemic agent which acts primarily by decreasing insulin resistance. Troglitazone is used in the management of type II diabetes (noninsulin-dependent diabetes mellitus (NIDDM) also known as adult-onset diabetes). It improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. Troglitazone is not chemically or functionally related to either the sulfonylureas, the biguanides, or the g-glucosidase inhibitors. Troglitazone may be used concomitantly with a sulfonylurea or insulin to improve glycemic control.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BG - Thiazolidinediones
A10BG01 - Troglitazone
Absorption
Absorbed rapidly. Food increases the extent of absorption by 30% to 85%.
A sulfate conjugate metabolite (Metabolite 1) and a quinone metabolite (Metabolite 3) have been detected in the plasma of healthy males. A glucuronide conjugate (Metabolite 2) has been detected in the urine and also in negligible amounts in the plasma. In healthy volunteers and in patients with type 2 diabetes, the steady-state concentration of Metabolite 1 is six to seven times that of troglitazone and Metabolite 3. In in vivo drug interaction studies, troglitazone has been shown to induce cytochrome P450 CYP3A4 at clinically relevant doses.
Troglitazone has known human metabolites that include (2S,3S,4S,5R)-6-[[2-[[4-[(2,4-dioxo-1,3-thiazolidin-5-yl)methyl]phenoxy]methyl]-2,5,7,8-tetramethyl-3,4-dihydrochromen-6-yl]oxy]-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
16-34 hours
Troglitazone is a thiazolidinedione antidiabetic agent that lowers blood glucose by improving target cell response to insulin. It has a unique mechanism of action that is dependent on the presence of insulin for activity. Troglitazone decreases hepatic glucose output and increases insulin dependent glucose disposal in skeletal muscle. Its mechanism of action is thought to involve binding to nuclear receptors (PPAR) that regulate the transcription of a number of insulin responsive genes critical for the control of glucose and lipid metabolism. Troglitazone is a ligand to both PPAR and PPAR, with a highter affinity for PPAR. The drug also contains an -tocopheroyl moiety, potentially giving it vitamin E-like activity. Troglitazone has been shown to reduce inflammation, and is associated with a decrase in nuclear factor kappa-B (NF-B) and a concomitant increase in its inhibitor (IB). Unlike sulfonylureas, troglitazone is not an insulin secretagogue.
