

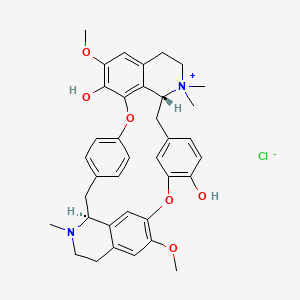

1. D-tubocurare
2. D-tubocurarine
3. Tubocurare
4. Tubocurarine
5. Tubocurarine Chloride
1. Tubocurarine Chloride
2. Sr-05000001878
3. 6989-98-6
4. 57-94-3
5. Chembl1687
6. Schembl41170
7. Spectrum1500602
8. Hms2092c04
9. Pharmakon1600-01500602
10. Ccg-39898
11. Nsc757362
12. Sr-05000001878-1
13. Sr-05000001878-3
| Molecular Weight | 645.2 g/mol |
|---|---|
| Molecular Formula | C37H41ClN2O6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 644.2653147 g/mol |
| Monoisotopic Mass | 644.2653147 g/mol |
| Topological Polar Surface Area | 80.6 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 990 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Neuromuscular Nondepolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction without causing depolarization of the motor end plate. They prevent acetylcholine from triggering muscle contraction and are used as muscle relaxants during electroshock treatments, in convulsive states, and as anesthesia adjuvants. (See all compounds classified as Neuromuscular Nondepolarizing Agents.)
Nicotinic Antagonists
Drugs that bind to nicotinic cholinergic receptors (RECEPTORS, NICOTINIC) and block the actions of acetylcholine or cholinergic agonists. Nicotinic antagonists block synaptic transmission at autonomic ganglia, the skeletal neuromuscular junction, and at central nervous system nicotinic synapses. (See all compounds classified as Nicotinic Antagonists.)