

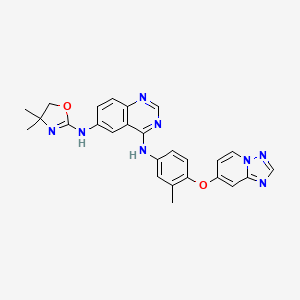

1. Irbinitinib
2. N6-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)-n4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine
3. N6-(4,5-dihydro-4,4-dmethyl-2-oxazolyl)-n4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-4,6-quinazolinediamine
4. Ont-380
5. Tukysa
1. Irbinitinib
2. 937263-43-9
3. Ont-380
4. Tukysa
5. 6-diamine
6. N6-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)-n4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine
7. 234248d0hh
8. Irbinitinib; Arry-380; Ont-380
9. 4,6-quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-n4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-
10. N4-(4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)-3-methylphenyl)-n6-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine
11. N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-n4-[3-methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-4,6-quinazolinediamine
12. Tucatinib [inn]
13. 6-n-(4,4-dimethyl-5h-1,3-oxazol-2-yl)-4-n-[3-methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]quinazoline-4,6-diamine
14. Unii-234248d0hh
15. Ont 380
16. Tukysa (tn)
17. Ont-380;tucatinib
18. Tucatinib [mi]
19. Tucatinib (usan/inn)
20. Tucatinib [usan:inn]
21. Tucatinib [usan]
22. Irbinitinib(arry-380)
23. Irbinitinib; Arry-380
24. Tucatinib [who-dd]
25. Gtpl9922
26. Schembl1193050
27. Tucatinib [orange Book]
28. Chembl3989868
29. Bdbm471617
30. Dtxsid601027958
31. Bcp15983
32. Ex-a1031
33. Mfcd22380467
34. Nsc764581
35. Nsc799335
36. S8362
37. Zinc68250462
38. Arry-380 (ont-380)
39. Akos026750449
40. Ccg-264747
41. Cs-3906
42. Db11652
43. Nsc-764581
44. Nsc-799335
45. Sb17126
46. Us10822334, Compound Ont380
47. Ncgc00482879-02
48. Ac-33037
49. As-56109
50. Bt177688
51. Hy-16069
52. Example 11 [wo2007059257a2]
53. Db-130430
54. A16413
55. D11141
56. A857335
57. Q25100690
58. 4,6-quinazolinediamine,n6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-n4-[3-methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-
59. N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-n4-[3-methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-4,6-quinazolinediamine;
60. N6-(4,5-dihydro-4,4-dmethyl-2-oxazolyl)-n4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-4,6-quinazolinediamine
| Molecular Weight | 480.5 g/mol |
|---|---|
| Molecular Formula | C26H24N8O2 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 480.20222204 g/mol |
| Monoisotopic Mass | 480.20222204 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 796 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tucatinib is indicated with trastuzumab and capecitabine for treatment of adults diagnosed with advanced unresectable or metastatic HER2-positive breast cancer. This includes patients with brain metastases and those who have received one or more prior anti-HER2-based regimens in the metastatic setting.
Tukysa is indicated in combination with trastuzumab and capecitabine for the treatment of adult patients with HER2positive locally advanced or metastatic breast cancer who have received at least 2 prior antiHER2 treatment regimens.
By inhibiting tyrosine kinase, tucatinib exerts anti-tumor activity, reducing the size of HER-2 positive breast cancer tumors. In clinical trials, the regimen of tucatinib and [trastuzumab] showed enhanced activity both in vitro and in vivo when compared to either drug administered by itself.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EH - Human epidermal growth factor receptor 2 (her2) tyrosine kinase inhibitors
L01EH03 - Tucatinib
Absorption
The Tmax for tucatinib ranges from 1 to 4 hours. One pharmacokinetic study revealed a Cmax of 1120 ng/mL after a dose of 350 mg twice daily with a Tmax ranging from 1 to 3 hours. The AUCtau was reported to be about 7120 hoursng/mL.
Route of Elimination
In a study of radiolabled tucatinib, about 86% of the total dose was excreted in the feces and 4.1% was found in the urine. About 16% of the tucatinib dose recovered in the feces was identified as unchanged tucatinib.
Volume of Distribution
The volume of distribution of tucatinib is about 1670 L. This drug penetrates the blood-brain barrier.
Clearance
The apparent clearance is 148 L/h.
Tucatinib is metabolized by CYP2C8 with some contributions from CYP3A.
A pharmacokinetic study revealed a half-life of approximately 5.38 hours. Prescribing information mentions a geometric mean half-life of about 8.21 hours.
Mutations in the HER-2 gene are observed in some types of breast carcinoma. Tucatinib inhibits the tyrosine kinase enzyme of the HER-2 gene. Mutations of tyrosine kinase in the HER-2 gene lead to cascade effects of increased cell signaling and proliferation, resulting in malignancy. Results of in vitro studies show that tucatinib inhibits the phosphorylation of both HER-2 and HER-3, leading to downstream changes in MAPK and AKT signaling and cell proliferation. Anti-tumor activity occured in the cells that expressed HER-2. In vivo, tucatinib has been shown to inhibit HER-2 expressing tumors, likely by the same mechanism.

