
1. 1650550-25-6
2. Ag-221 Mesylate
3. Enasidenib Mesilate
4. Enasidenib Methanesulfonate
5. Enasidenib (mesylate)
6. Uf6pc17xav
7. Enasidenib Mesylate [usan]
8. Cc-90007
9. 1650550-25-6 (mesylate)
10. Enasidenib Mesylate (usan)
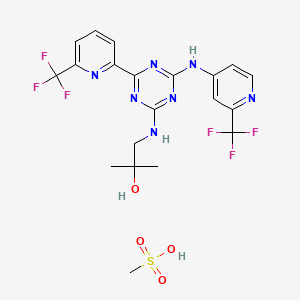
11. 2-propanol, 2-methyl-1-((4-(6-(trifluoromethyl)-2-pyridinyl)-6-((2-(trifluoromethyl)-4-pyridinyl)amino)-1,3,5-triazin-2-yl)amino)-, Methanesulfonate (1:1)
12. 2-methyl-1-[4-(6-trifluoromethyl-pyridin-2-yl)-6-(2-trifluoromethyl-pyridin-4-ylamino)-[1,3,5]triazin-2-ylamino]-propan-2-ol Mesylate
13. Methanesulfonic Acid;2-methyl-1-[[4-[6-(trifluoromethyl)pyridin-2-yl]-6-[[2-(trifluoromethyl)pyridin-4-yl]amino]-1,3,5-triazin-2-yl]amino]propan-2-ol
14. Unii-uf6pc17xav
15. Idhifa (tn)
16. Chembl3989931
17. Enasidenib Mesylate [mi]
18. Schembl16448052
19. Dtxsid501027943
20. Bcp26166
21. Ex-a1972
22. Enasidenib Mesylate [who-dd]
23. Hy-18690a
24. S4929
25. Enasidenib Mesylate; Ag-221 Mesylate
26. At36098
27. Cs-7541
28. Enasidenib Mesylate [orange Book]
29. Ac-31319
30. D11044
31. Q27291054
32. 2-methyl-1-((4-(6-(trifluoromethyl)pyridin-2-yl)- 6-((2-(trifluoromethyl)pyridin-4-yl)amino)-1,3,5-triazin- 2-yl)amino)propan-2-ol Methanesulfonate
| Molecular Weight | 569.5 g/mol |
|---|---|
| Molecular Formula | C20H21F6N7O4S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 6 |
| Exact Mass | 569.12799232 g/mol |
| Monoisotopic Mass | 569.12799232 g/mol |
| Topological Polar Surface Area | 172 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 727 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |