

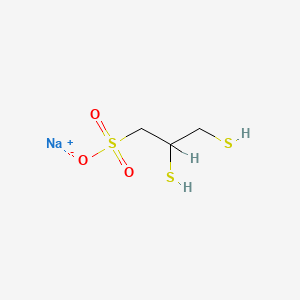

1. 1-sodium Sulfonate, 2,3-dimercaptopropane
2. 2,3 Dimercapto 1 Propanesulfonic Acid
3. 2,3 Dimercaptopropane 1 Sodium Sulfonate
4. 2,3 Dimercaptopropane 1 Sulfonate Sodium
5. 2,3 Dimercaptopropanesulfonic Acid
6. 2,3-dimercapto-1-propanesulfonic Acid
7. 2,3-dimercaptopropane 1-sodium Sulfonate
8. 2,3-dimercaptopropane Sulfonate, Sodium
9. 2,3-dimercaptopropane-1-sulfonate Sodium
10. 2,3-dimercaptopropanesulfonic Acid
11. 2,3-dithiopropanesulfate, Sodium
12. Dimaval
13. Dmps
14. Dmps Heyl
15. Dmps-heyl
16. Mercuval
17. Sodium 2,3-dimercaptopropane Sulfonate
18. Sodium 2,3-dithiopropanesulfate
19. Unitiol
1. 4076-02-2
2. Sodium 2,3-dimercapto-1-propanesulfonate
3. Sodium 2,3-dimercaptopropane-1-sulfonate
4. Dmps
5. Unitiol
6. Dimaval
7. Sodium 2,3-dimercaptopropanesulfonate
8. 1-propanesulfonic Acid, 2,3-dimercapto-, Monosodium Salt
9. Sodium 2,3-dimercaptopropanesulphonate
10. 2,3-dimercapto-1-propanesulfonic Acid Sodium Salt
11. 690vn2l7tk
12. Sodium;2,3-bis(sulfanyl)propane-1-sulfonate
13. Sodium 2,3-disulfanylpropane-1-sulfonate
14. 2,3-dimercaptopropane-1-sulfonate Sodium
15. Sodium 2,3-dithiolpropanesulfonate
16. Einecs 223-796-3
17. 2,3-dimercaptopropane Sodium Sulphonate
18. (+)-dmps
19. (-)-dmps
20. Sodium 2,3-bis(sulfanyl)propane-1-sulfonate
21. Unithiol [mart.]
22. Unithiol [who-dd]
23. 2,3-dimercaptopropanesulfonic Acid Sodium Salt
24. Dmps (sodium Salt)
25. Unii-690vn2l7tk
26. Schembl164318
27. Niosh/tz6420050
28. Niosh/tz6420100
29. Sodium Dimercaptopropane Sulfonate
30. Dtxsid40958410
31. Amy22488
32. Mfcd00007523
33. Stl372655
34. Akos015898653
35. Akos015967317
36. Sodium 2,3-dimercapto-sulfonate
37. Sodium Dimercaptopropanesulphonate
38. As-64437
39. Sodium Dimercaptopropane Sulphonate
40. Db-049647
41. Ft-0609675
42. Tz64200500
43. Tz64201000
44. H10946
45. 2,3-dimercaptopropane-1-sulphonate Sodium
46. A923350
47. Dimercaptopropane Sulfonic Acid Sodium Salt
48. Sodium 2,3-dimercapto-1-propanesulphonate
49. (+)-2,3-dimercapto-1-propanesulfonate Sodium Salt
50. (-)-2,3-dimercapto-1-propanesulfonate Sodium Salt
51. D-2,3-dimercapto-1-propanesulfonic Acid Sodium Salt
52. L-2,3-dimercapto-1-propanesulfonic Acid Sodium Salt
53. Q-201912
54. Q26841293
55. 1-propanesulfonic Acid, 2,3-dimercapto-, Sodium Salt, (+)-
56. 1-propanesulfonic Acid, 2,3-dimercapto-, Sodium Salt, (-)-
57. 2,3-dimercapto-1-propanesulfonic Acid Sodium Salt [mi]
58. 1-propanesulfonic Acid, 2,3-dimercapto-, Sodium Salt (1:1)
59. 37260-06-3
| Molecular Weight | 210.3 g/mol |
|---|---|
| Molecular Formula | C3H7NaO3S3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 209.94550188 g/mol |
| Monoisotopic Mass | 209.94550188 g/mol |
| Topological Polar Surface Area | 67.6 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)