
1. Calan
2. Cordilox
3. Dexverapamil
4. Falicard
5. Finoptin
6. Hydrochloride, Verapamil
7. Iproveratril
8. Isoptin
9. Isoptine
10. Izoptin
11. Lekoptin
12. Verapamil Hydrochloride
1. 52-53-9
2. Iproveratril
3. Vasolan
4. Dilacoran
5. Isoptin
6. Falicard
7. Finoptin
8. Isoptine
9. Calan
10. Lekoptin
11. Verapamilo
12. Verapamilum
13. Isotopin
14. Securon
15. Calcan
16. Tarka
17. D-365
18. Cp-16533-1
19. Cardibeltin
20. Izoptin
21. Verelan
22. Covera-hs
23. Verpamil
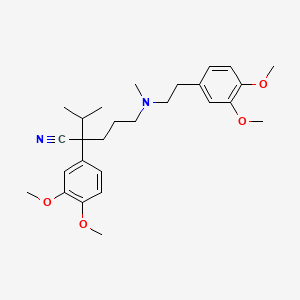
24. 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile
25. Verelan Pm
26. 5-((3,4-dimethoxyphenethyl)(methyl)amino)-2-(3,4-dimethoxyphenyl)-2-isopropylpentanenitrile
27. 5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile
28. Cardiagutt
29. Berkatens
30. Dignover
31. Geangin
32. Veramex
33. Quasar
34. Univer
35. Novo-veramil
36. Verapamil Slow Release
37. Apo-verap
38. Isoptin Sr
39. Nu-verap
40. Calan Sr
41. Isoptimo
42. Benzeneacetonitrile, Alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)methylamino)propyl)-3,4-dimethoxy-alpha-(1-methylethyl)-
43. (+/-)-verapamil Hydrochlorid
44. 2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-propan-2-ylpentanenitrile
45. Cj0o37ku29
46. Chebi:77733
47. Cp-16,533-1
48. Cardiabeltin
49. Cardioprotect
50. Durasoptin
51. Hexasoptin
52. Veratensin
53. Veroptinstada
54. Calaptin
55. Caveril
56. Civicor
57. Coraver
58. Corpamil
59. Harteze
60. Hormitol
61. Ikapress
62. Inselon
63. Isoptino
64. Jenapamil
65. Lodixal
66. Magotiron
67. Praecicor
68. Robatelan
69. Vasomil
70. Vasopten
71. Verabeta
72. Veracor
73. Verahexal
74. Veraloc
75. Veramil
76. Verapin
77. Verasal
78. Verasifar
79. Verdilac
80. Vetrimil
81. Akilen
82. Elthon
83. Flamon
84. Ikacor
85. Univex
86. Vortac
87. Anpec
88. Ormil
89. Rapam
90. Civicor Retard
91. Isoptin Retard
92. Manidon Retard
93. Valeronitrile, 5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropyl-
94. Vera-sanorania
95. Verapamil Acis
96. Verapamil Atid
97. Verapamil Ebewe
98. Verapamil Riker
99. Verapamil Verla
100. (+/-)-verapamil;cp-16533-1
101. Verapamil Basics
102. Verapamil Nordic
103. Verapamil-abz
104. Novapamyl Lp
105. Verapamil Al
106. Verapamil Nm
107. Verapamil Pb
108. Verapamil Sr
109. Cordilox Sr
110. Dilacoran Hta
111. Veracaps Sr
112. Verapamil Msd
113. Verapamilum [inn-latin]
114. Arpamyl Lp
115. Hexasoptin Retard
116. Verapamil Henning
117. Verelan Sr
118. Ncgc00016083-09
119. Verapamilo [inn-spanish]
120. Cp-165331
121. Verapamil Injection
122. (+/-)-verapamil
123. Verapamil Nm Pharma
124. Calaptin 240 Sr
125. Verapress 240 Sr
126. 5-[(3,4-dimethoxyphenethyl)methylamino]-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile
127. Dsstox_cid_21152
128. Dsstox_rid_79636
129. Dsstox_gsid_41152
130. Benzeneacetonitrile, .alpha.-[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-.alpha.-(1-methylethyl)-
131. Verapamil [usan:inn:ban]
132. Dl-verapamil
133. R,s-verapamil
134. Cas-52-53-9
135. Ccris 6749
136. Nsc272366
137. Verapamil (usan/inn)
138. Einecs 200-145-1
139. Unii-cj0o37ku29
140. Ansyr
141. Nsc 272306na
142. Einecs 260-462-6
143. Calan (salt/mix)
144. Akilen (salt/mix)
145. Alpha-((n-methyl-n-homoveratryl)-gamma-aminopropyl)-3,4-dimethoxyphenylacetonitrile
146. Isoptin (salt/mix)
147. Alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)-methylamino)propyl)-3,4-dimethoxy-alpha-(1-methylethyl)benzeneacetonitrile
148. Alpha-isopropyl-alpha-((n-methyl-n-homoveratryl)-gamma-aminopropyl)-3,4-dimethoxyphenylacetonitrile
149. Cordilox (salt/mix)
150. Delta-365
151. Verapamil [inn]
152. Verapamil [mi]
153. Covera-hs (salt/mix)
154. Verapamil [usan]
155. Prestwick0_000141
156. Prestwick1_000141
157. Prestwick2_000141
158. Prestwick3_000141
159. Spectrum2_001740
160. Spectrum4_000906
161. Spectrum5_001786
162. Verapamil [vandf]
163. Cp 16533-1
164. D 365
165. Verapamil [who-dd]
166. Chembl6966
167. Lopac0_001237
168. Schembl16742
169. Bspbio_000242
170. Bspbio_001513
171. Bspbio_002358
172. Kbiogr_000233
173. Kbiogr_001372
174. Kbiogr_002343
175. Kbioss_000233
176. Kbioss_002346
177. 56949-77-0
178. Benzeneacetonitrile, Alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)methylamino) Propyl)-3,4-dimethoxy-alpha-(1-methylethyl)-
179. Mls006011414
180. Divk1c_000399
181. Spbio_001820
182. Spbio_002181
183. Bpbio1_000268
184. Gtpl2406
185. Cp 16533-1 (verapamil)
186. Dtxsid9041152
187. Schembl13287282
188. (a+/-)-verapamil Hydrochloride
189. Bdbm81939
190. Hsdb 3928
191. Kbio1_000399
192. Kbio2_000233
193. Kbio2_002343
194. Kbio2_002801
195. Kbio2_004911
196. Kbio2_005369
197. Kbio2_007479
198. Kbio3_000465
199. Kbio3_000466
200. Kbio3_002823
201. Cmap_000023
202. Ninds_000399
203. Nsc-272306na
204. Bio1_000425
205. Bio1_000914
206. Bio1_001403
207. Bio2_000233
208. Bio2_000713
209. Hms1791l15
210. Hms1989l15
211. Hms2089h17
212. Hms3402l15
213. Tox21_110300
214. Mfcd00056240
215. Nsc_62969
216. Stk538085
217. Akos005468962
218. Tox21_110300_1
219. Ccg-205311
220. Db00661
221. Sdccgsbi-0051204.p005
222. Cas_52-53-9
223. Idi1_000399
224. Idi1_033983
225. Ncgc00016083-04
226. Ncgc00016083-05
227. Ncgc00016083-06
228. Ncgc00016083-07
229. Ncgc00016083-08
230. Ncgc00016083-10
231. Ncgc00016083-11
232. Ncgc00016083-13
233. Ncgc00016083-14
234. Ncgc00016083-15
235. Ncgc00016083-16
236. Ncgc00016083-17
237. Ncgc00016083-18
238. Ncgc00016083-20
239. Ncgc00016083-25
240. Ncgc00016083-34
241. Ncgc00024710-04
242. Ncgc00024710-05
243. Ncgc00024710-06
244. Ncgc00024710-07
245. Ncgc00024710-08
246. Ncgc00024710-09
247. Ncgc00344584-01
248. 2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methyl-amino]-2-(1-methylethyl) Pentanenitrile
249. 2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methyl-amino]-2-isopropyl-pentanenitrile
250. Ac-16016
251. Bp-21223
252. Hy-14275
253. Nci60_020143
254. Smr001550201
255. Sbi-0051204.p003
256. Ab00053495
257. Cs-0002967
258. Ft-0603225
259. Ft-0675801
260. 52v114
261. C07188
262. D02356
263. E75969
264. Ab00053495-20
265. Ab00053495_21
266. A829133
267. L001330
268. Q410291
269. Brd-a09533288-001-02-7
270. Brd-a09533288-003-05-6
271. Cp-165331 / Cp-16533-1
272. F2173-0851
273. Verapamil, Dexverapamil, Verapamyl Hydrochloride, Verapamil Hydrochloride
274. .alpha.-((n-methyl-n-homoveratryl)-.gamma.-aminopropyl)-3,4-dimethoxyphenylacetonitrile
275. (+/-)-5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile
276. (?)-alpha-[3-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)benzeneacetonitrile
277. 2-(3,4-dimethoxyphenyl)-5-((2-(3,4-dimethoxyphenyl)ethyl)(methyl)amino)-2-isopropylpentanenitrile, (+/-)-
278. 2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]-2-isopropylpentanenitrile #
| Molecular Weight | 454.6 g/mol |
|---|---|
| Molecular Formula | C27H38N2O4 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 13 |
| Exact Mass | 454.28315770 g/mol |
| Monoisotopic Mass | 454.28315770 g/mol |
| Topological Polar Surface Area | 64 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Calan |
| PubMed Health | Verapamil (By mouth) |
| Drug Classes | Antianginal, Antiarrhythmic, Group IV, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | CALAN (verapamil HCl) is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) available for oral administration in film-coated tablets containing 40 mg, 80 mg, or 120 mg of verapamil hydrochloride.The structural formula of... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 120mg; 80mg; 40mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 2 of 12 | |
|---|---|
| Drug Name | Calan sr |
| PubMed Health | Verapamil |
| Drug Classes | Antianginal, Antiarrhythmic, Group IV, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | CALAN SR (verapamil hydrochloride) is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist). CALAN SR is available for oral administration as light green, capsule-shaped, scored, film-coated tablets (caplets) containing 240... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Pfizer |
| 3 of 12 | |
|---|---|
| Drug Name | Covera-hs |
| PubMed Health | Trandolapril/Verapamil (By mouth) |
| Drug Classes | ACE Inhibitor/Calcium Channel Blocker Combination |
| Drug Label | COVERA-HS (verapamil hydrochloride) is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist). COVERA-HS is available for oral administration as pale yellow, round, film-coated tablets containing 240 mg of verapamil hydrochlo... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 180mg; 240mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 4 of 12 | |
|---|---|
| Drug Name | Tarka |
| Active Ingredient | verapamil hydrochloride; Trandolapril |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 180mg; 1mg; 4mg; 2mg; 240mg |
| Market Status | Prescription |
| Company | Abbvie |
| 5 of 12 | |
|---|---|
| Drug Name | Verelan |
| Drug Label | Verelan (verapamil hydrochloride capsules) is a calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist). Verelan is available for oral administration as a 360 mg hard gelatin capsule (lavender cap/yellow body), a 240 mg hard g... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Alkermes Gainesville |
| 6 of 12 | |
|---|---|
| Drug Name | Verelan pm |
| Drug Label | Verelan PM (verapamil hydrochloride) is a calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist). Verelan PM is available for oral administration as a 100 mg hard gelatin capsule (white opaque cap/amethyst body), a 200 mg hard... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 300mg; 100mg |
| Market Status | Prescription |
| Company | Alkermes Gainesville |
| 7 of 12 | |
|---|---|
| Drug Name | Calan |
| PubMed Health | Verapamil (By mouth) |
| Drug Classes | Antianginal, Antiarrhythmic, Group IV, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | CALAN (verapamil HCl) is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) available for oral administration in film-coated tablets containing 40 mg, 80 mg, or 120 mg of verapamil hydrochloride.The structural formula of... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 120mg; 80mg; 40mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 8 of 12 | |
|---|---|
| Drug Name | Calan sr |
| PubMed Health | Verapamil |
| Drug Classes | Antianginal, Antiarrhythmic, Group IV, Antihypertensive, Antimigraine, Cardiovascular Agent |
| Drug Label | CALAN SR (verapamil hydrochloride) is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist). CALAN SR is available for oral administration as light green, capsule-shaped, scored, film-coated tablets (caplets) containing 240... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 240mg |
| Market Status | Prescription |
| Company | Pfizer |
| 9 of 12 | |
|---|---|
| Drug Name | Covera-hs |
| PubMed Health | Trandolapril/Verapamil (By mouth) |
| Drug Classes | ACE Inhibitor/Calcium Channel Blocker Combination |
| Drug Label | COVERA-HS (verapamil hydrochloride) is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist). COVERA-HS is available for oral administration as pale yellow, round, film-coated tablets containing 240 mg of verapamil hydrochlo... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 180mg; 240mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 10 of 12 | |
|---|---|
| Drug Name | Tarka |
| Active Ingredient | verapamil hydrochloride; Trandolapril |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 180mg; 1mg; 4mg; 2mg; 240mg |
| Market Status | Prescription |
| Company | Abbvie |
| 11 of 12 | |
|---|---|
| Drug Name | Verelan |
| Drug Label | Verelan (verapamil hydrochloride capsules) is a calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist). Verelan is available for oral administration as a 360 mg hard gelatin capsule (lavender cap/yellow body), a 240 mg hard g... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 180mg; 120mg; 360mg; 240mg |
| Market Status | Prescription |
| Company | Alkermes Gainesville |
| 12 of 12 | |
|---|---|
| Drug Name | Verelan pm |
| Drug Label | Verelan PM (verapamil hydrochloride) is a calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist). Verelan PM is available for oral administration as a 100 mg hard gelatin capsule (white opaque cap/amethyst body), a 200 mg hard... |
| Active Ingredient | Verapamil hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 300mg; 100mg |
| Market Status | Prescription |
| Company | Alkermes Gainesville |
Anti-Arrhythmia Agents; Calcium Channel Blockers; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2017)
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Verapamil hydrochloride is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of April 18, 2017: https://clinicaltrials.gov/
Oral calcium-channel blocking agents are considered the drugs of choice for the management of Prinzmetal variant angina. A nondihydropyridine calcium-channel blocker (e.g., diltiazem, verapamil) also has been recommended in patients with unstable angina who have continuing or ongoing ischemia when therapy with beta-blocking agents and nitrates is inadequate, not tolerated, or contraindicated and when severe left ventricular dysfunction, pulmonary edema, or other contraindications are not present. In the management of unstable or chronic stable angina pectoris, verapamil appears to be as effective as beta-adrenergic blocking agents (e.g., propranolol) and/or oral nitrates. In unstable or chronic stable angina pectoris, verapamil may reduce the frequency of attacks, allow a decrease in sublingual nitroglycerin dosage, and increase the patient's exercise tolerance. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2059
Verapamil is used for rapid conversion to sinus rhythm of paroxysmal supraventricular tachycardia (PSVT), including tachycardia associated with Wolff-Parkinson-White or Lown-Ganong-Levine syndrome; the drug also is used for control of rapid ventricular rate in nonpreexcited atrial flutter or fibrillation. The American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guideline for the management of adult patients with supraventricular tachycardia recommends the use of verapamil in the treatment of various SVTs (e.g., atrial flutter, junctional tachycardia, focal atrial tachycardia, atrioventricular nodal reentrant tachycardia (AVNRT)); in general, IV verapamil is recommended for acute treatment, while oral verapamil is recommended for ongoing management of these arrhythmias. /Included in the US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2059
For more Therapeutic Uses (Complete) data for Verapamil (14 total), please visit the HSDB record page.
...Concurrent treatment /of verapamil & beta-blockers/ in those with impaired left ventricular function could be dangerous if...a 10-15% depression in myocardial function takes place. /Salt not specified/
Stockley, I.H. Drug Interactions. Boston: Blackwell Scientific Publications, 1981., p. 68
...Absolute contraindications to the use of verapamil (the acute stage of myocardial infarction, complete atrioventricular block, cardiogenic shock, overt heart failure)...should not be injected together with a beta-adrenergic blocking agent, or within 3 times the half-life of that agent. /Salt not specified/
Stockley, I.H. Drug Interactions. Boston: Blackwell Scientific Publications, 1981., p. 68
The basic physiologic actions of verapamil may lead to serious adverse effects. /Salt not specified/
Epstein SE, Rosing DR; Circulation 64 (3): 37-41 (1981)
Maternal Medication usually Compatible with Breast-Feeding: Verapamil: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/ /Salt not specified/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 142 (1994)
For more Drug Warnings (Complete) data for Verapamil (23 total), please visit the HSDB record page.
Verapamil is indicated in the treatment of vasopastic (i.e. Prinzmetal's) angina, unstable angina, and chronic stable angina. It is also indicated to treat hypertension, for the prophylaxis of repetitive paroxysmal supraventricular tachycardia, and in combination with digoxin to control ventricular rate in patients with atrial fibrillation or atrial flutter. Given intravenously, it is indicated for the treatment of various supraventricular tachyarrhythmias, including rapid conversion to sinus rhythm in patients with supraventricular tachycardia and for temporary control of ventricular rate in patients with atrial fibrillation or atrial flutter. Verapamil is commonly used off-label for prophylaxis of cluster headaches.
Verapamil is an L-type calcium channel blocker with antiarrhythmic, antianginal, and antihypertensive activity. Immediate-release verapamil has a relatively short duration of action, requiring dosing 3 to 4 times daily, but extended-release formulations are available that allow for once-daily dosing. As verapamil is a negative inotropic medication (i.e. it decreases the strength of myocardial contraction), it should not be used in patients with severe left ventricular dysfunction or hypertrophic cardiomyopathy as the decrease in contractility caused by verapamil may increase the risk of exacerbating these pre-existing conditions.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C08DA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C08DA01
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C - Cardiovascular system
C08 - Calcium channel blockers
C08D - Selective calcium channel blockers with direct cardiac effects
C08DA - Phenylalkylamine derivatives
C08DA01 - Verapamil
Absorption
More than 90% of orally administered verapamil is absorbed - despite this, bioavailability ranges only from 20% to 30% due to rapid biotransformation following first-pass metabolism in the portal circulation. Absorption kinetic parameters are largely dependent on the specific formulation of verapamil involved. Immediate-release verapamil reaches peak plasma concentrations (i.e. Tmax) between 1-2 hours following administration, whereas sustained-release formulations tend to have a Tmax between 6 - 11 hours. AUC and Cmax values are similarly dependent upon formulation. Chronic administration of immediate-release verapamil every 6 hours resulted in plasma concentrations between 125 and 400 ng/mL. Steady-state AUC0-24h and Cmax values for a sustained-release formulation were 1037 ngh/ml and 77.8 ng/mL for the R-isomer and 195 ngh/ml and 16.8 ng/mL for the S-isomer, respectively. Interestingly, the absorption kinetics of verapamil are highly stereospecific - following oral administration of immediate-release verapamil every 8 hours, the relative systemic availability of the S-enantiomer compared to the R-enantiomer was 13% after a single dose and 18% at steady-state.
Route of Elimination
Approximately 70% of an administered dose is excreted as metabolites in the urine and 16% in the feces within 5 days. Approximately 3% - 4% is excreted in the urine as unchanged drug.
Volume of Distribution
Verapamil has a steady-state volume of distribution of approximately 300L for its R-enantiomer and 500L for its S-enantiomer.
Clearance
Systemic clearance following 3 weeks of continuous treatment was approximately 340 mL/min for R-verapamil and 664 mL/min for S-verapamil. Of note, apparent oral clearance appears to vary significantly between single dose and multiple-dose conditions. The apparent oral clearance following single doses of verapamil was approximately 1007 mL/min for R-verapamil and 5481 mL/min for S-verapamil, whereas 3 weeks of continuous treatment resulted in apparent oral clearance values of approximately 651 mL/min for R-verapamil and 2855 mL/min for S-verapamil.
/MILK/ Breast milk: Verapamil may appear in breast milk.
International Programme on Chemical Safety; Poisons Information Monograph: Verapamil (PIM 552) (1991) Available from, as of April 7, 2009: https://www.inchem.org/pages/pims.html
/MILK/ Verapamil is excreted into breast milk. A daily dose of 240 mg produced milk levels that were approx 23% of maternal serum. Serum levels in the infant were 2.1 ng/mL but could not be detected (<1 ng/mL) 38 hr after treatment was stopped. ... In a second case, a mother was treated with 80 mg 3 times/day for hypertension for 4 wk prior to the determination of serum & milk concns. Steady-state concentrations of verapamil and the metabolite, norverapamil, in milk were 25.8 and 8.8 ng/mL, respectively. These values were 60% and 16% of the concns in plasma. The investigators estimated that the breast-fed child received <0.01% of the mother's dose. Neither verapamil nor the metabolite could be detected in the plasma of the child.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 879
The pharmacokinetics and hemodynamic effects of a combination of verapamil and trandolapril were studied in 20 patients with hypertension (ages 29-71 yr), 10 of whom also had fatty liver disease, who received a sustained-release oral capsule containing 180 mg verapamil and 1 mg trandolapril once daily for 7 days. For verapamil, no statistically significant differences were seen between patients with and without fatty liver with regard to Cmax (110.5 vs 76.5 ug/L), plasma AUC from 0-24 hr (1260.6 vs 941.2 ug/L hr), and elimination half-life (9.8 vs 9.2 hr).
Siepmann M et al; Clin Drug Invest 14 : 376-382 (1997)
An open, randomized, single dose study of the effects of food on the bioavailability of sustained-release (SR) verapamil hydrochloride (Isoptin) was conducted in 12 healthy volunteers (aged 19-65 yr) who received 240 mg of the SR preparation while fasting or with food and a conventional preparation while fasting. Although the elimination half-life of SR verapamil was unchanged, the time to maximum concentration was prolonged and the area under the concentration-time curve (AUC) was 80% of the regular preparation. Concomitant food administration prolonged the time to maximum concentration from 7.3+-3.4 to 11.7+-6.3 h but had little effect on the maximum concentration, half-life or AUC of SR verapamil.
PMID:2338632 Conway EL et al; J Pharm Sci 79 : 228-231 (1990)
For more Absorption, Distribution and Excretion (Complete) data for Verapamil (21 total), please visit the HSDB record page.
Verapamil is extensively metabolized by the liver, with up to 80% of an administered dose subject to elimination via pre-systemic metabolism - interestingly, this first-pass metabolism appears to clear the S-enantiomer of verapamil much faster than the R-enantiomer. The remaining parent drug undergoes O-demethylation, N-dealkylation, and N-demethylation to a number of different metabolites via the cytochrome P450 enzyme system. Norverapamil, one of the major circulating metabolites, is the result of verapamil's N-demethylation via CYP2C8, CYP3A4, and CYP3A5, and carries approximately 20% of the cardiovascular activity of its parent drug. The other major pathway involved in verapamil metabolism is N-dealkylation via CYP2C8, CYP3A4, and CYP1A2 to the D-617 metabolite. Both norverapamil and D-617 are further metabolized by other CYP isoenzymes to various secondary metabolites. CYP2D6 and CYP2E1 have also been implicated in the metabolic pathway of verapamil, albeit to a minor extent. Minor pathways of verapamil metabolism involve its O-demethylation to D-703 via CYP2C8, CYP2C9, and CYP2C18, and to D-702 via CYP2C9 and CYP2C18. Several steps in verapamil's metabolic pathway show stereoselective preference for the S-enantiomer of the given substrate, including the generation of the D-620 metabolite by CYP3A4/5 and the D-617 metabolite by CYP2C8.
Metabolites: The main metabolite is norverapamil which has an elimination half-life very similar to that of the parent compound, ranging from 4 to 8 hours. Verapamil undergoes an extensive hepatic metabolism. Due to a large hepatic first-pass effect, bioavailability does not exceed 20 - 35% in normal subjects. Twelve metabolites have been described. The main metabolite is norverapamil and the others are various N- and 0-dealkylated metabolites. Elimination by route of exposure: Kidney: About 70% of the administered dose is excreted in urine within 5 days as metabolites, of which 3-4% is excreted as unchanged drug. Feces: About 16% of the ingested dose is excreted within 5 days in feces as metabolites. Breast milk: Verapamil may appear in breast milk.
International Programme on Chemical Safety; Poisons Information Monograph: Verapamil (PIM 552) (1991) Available from, as of April 7, 2009: https://www.inchem.org/pages/pims.html
Verapamil yields in the dog: 5-(3,4-dimethoxyphenethylamino)-2 -(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile; 2-(3,4-dimethoxyphenyl)-5 -(n-(4-hydroxy-3-methoxyphenethyl)methylamino)-2-isopropylvaleronitrile, and 2-(3,4-dimethoxyphenyl)-2-isopropyl-5-methylaminovaleronitrile. The latter was also found in rats. /From table/ /salt not specified/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. D-79
Verapamil and its major metabolite norverapamil were identified to be both mechanism-based inhibitors and substrates of CYP3A and reported to have non-linear pharmacokinetics in clinic. Metabolic clearances of verapamil and norverapmil as well as their effects on CYP3A activity were firstly measured in pooled human liver microsomes. The results showed that S-isomers were more preferential to be metabolized than R-isomers for both verapamil and norverapamil, and their inhibitory effects on CYP3A activity were also stereoselective with S-isomers more potent than R-isomers. A semi-physiologically based pharmacokinetic model (semi-PBPK) characterizing mechanism-based auto-inhibition was developed to predict the stereoselective pharmacokinetic profiles of verapamil and norverapamil following single or multiple oral doses. Good simulation was obtained, which indicated that the developed semi-PBPK model can simultaneously predict pharmacokinetic profiles of S-verapamil, R-verapamil, S-norverapamil and R-norverapamil. Contributions of auto-inhibition to verapamil and norverapamil accumulation were also investigated following the 38th oral dose of verapamil sustained-release tablet (240 mg once daily). The predicted accumulation ratio was about 1.3-1.5 fold, which was close to the observed data of 1.4-2.1-fold. Finally, the developed semi-PBPK model was further applied to predict drug-drug interactions (DDI) between verapamil and other three CYP3A substrates including midazolam, simvastatin, and cyclosporine A. Successful prediction was also obtained, which indicated that the developed semi-PBPK model incorporating auto-inhibition also showed great advantage on DDI prediction with CYP3A substrates.
PMID:23916407 Wang J et al; Eur J Pharm Sci 50 (3-4): 290-302 (2013)
The biotransformation pathway of verapamil, a widely prescribed calcium channel blocker, was investigated by electrochemistry (EC) coupled online to liquid chromatography (LC) and electrospray mass spectrometry (ESI-MS). Mimicry of the oxidative phase I metabolism was achieved in a simple amperometric thin-layer cell equipped with a boron-doped diamond (BDD) working electrode. Structures of the electrochemically generated metabolites were elucidated on the basis of accurate mass data and additional MS/MS experiments. We were able to demonstrate that all of the most important metabolic products of the calcium antagonist including norverapamil (formed by N-demethylation) can easily be simulated using this purely instrumental technique. Furthermore, newly reported metabolic reaction products like carbinolamines or imine methides become accessible. The results obtained by EC were compared with conventional in vitro studies by conducting incubations with rat as well as human liver microsomes (RLMs, HLMs). Both methods showed good agreement with the data from EC/LC/MS. Thus, it can be noted that EC is very well-suited for the simulation of the oxidative metabolism of verapamil. In summary, this study confirms that EC/LC/MS can be a powerful tool in drug discovery and development when applied complementary to established in vitro or in vivo approaches.
PMID:22098935 Jahn S et al; J Chromatogr A 1218 (51): 9210-20 (2011)
Mechanism-based inactivation (MBI) of cytochrome P450 (CYP) 3A by verapamil and the resulting drug-drug interactions have been studied in vitro, but the inhibition of verapamil on its own metabolic clearance in clinic, namely auto-inhibition of verapamil metabolism, has never been reproduced in vitro. This paper aimed to evaluate the utility of gel entrapped rat hepatocytes in reflecting such metabolic auto-inhibition using hepatocyte monolayer as a control. Despite being a similar concentration- and time-dependent profile, auto-inhibition of verapamil metabolism showed apparent distinctions between the two culture models. Firstly, gel entrapped hepatocytes were more sensitive to such inhibition, which could be largely due to their higher CYP3A activity detected by the formation rates of 6-beta-hydroxy testosterone and 1'-hydroxy midazolam. Furthermore, the inhibitory effect of ketoconazole and verapamil on CYP 3A activity as well as the reduction of verapamil intrinsic clearance (CL(int)) by ketoconazole was only observed in gel-entrapped hepatocytes. In this respect, the involvement of CYP3A in auto-inhibition of verapamil metabolism could be illustrated in gel-entrapped hepatocytes but not in hepatocyte monolayer. All of these results indicated that hepatocytes of gel entrapment reflected more of verapamil metabolic auto-inhibition than hepatocyte monolayer and could serve as a suitable system for investigating drug metabolism.
PMID:21775094 Yin J et al; Biomed Pharmacother 65 (5): 328-33 (2011)
Verapamil has known human metabolites that include 2-(3,4-dimethoxyphenyl)acetaldehyde, D-617, D-702, M9 (D-703), and Norverapamil.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Single-dose studies of immediate-release verapamil have demonstrated an elimination half-life of 2.8 to 7.4 hours, which increases to 4.5 to 12.0 hours following repetitive dosing. The elimination half-life is also prolonged in patients with hepatic insufficiency (14 to 16 hours) and in the elderly (approximately 20 hours). Intravenously administered verapamil has rapid distribution phase half-life of approximately 4 minutes, followed by a terminal elimination phase half-life of 2 to 5 hours.
The pharmacokinetics of verapamil and its metabolite, norverapamil, were studied in 10 patients (ages 19-69 yr) with portal hypertension and in 6 healthy subjects (ages 21-69 yr) who received an oral dose of 80 mg verapamil hydrochloride (Isoptin). The terminal phase half-life of verapamil was 210 hr in controls and 1384 hr in patients.
Vlcek J et al; Arzneim Forsch 45(2) : 146-149 (1995)
A toxicokinetic study performed in two cases showed plasma half lives of 7.9 and 13.2 hours, total body clearances of 425 and 298 mL/min. ...
PMID:2262922 Sauder P et al; J Toxicol Clin Exp 10(4) : 261-70 (1990)
Verapamil inhibits L-type calcium channels by binding to a specific area of their alpha-1 subunit,Cav1.2, which is highly expressed on L-type calcium channels in vascular smooth muscle and myocardial tissue where these channels are responsible for the control of peripheral vascular resistance and heart contractility. Calcium influx through these channels allows for the propagation of action potentials necessary for the contraction of muscle tissue and the heart's electrical pacemaker activity. Verapamil binds to these channels in a voltage- and frequency-dependent manner, meaning affinity is increased 1) as vascular smooth muscle membrane potential is reduced, and 2) with excessive depolarizing stimulus. Verapamil's mechanism of action in the treatment of angina and hypertension is likely due to the mechanism described above. Inhibition of calcium influx prevents the contraction of vascular smooth muscle, causing relaxation/dilation of blood vessels throughout the peripheral circulation - this lowers systemic vascular resistance (i.e. afterload) and thus blood pressure. This reduction in vascular resistance also reduces the force against which the heart must push, decreasing myocardial energy consumption and oxygen requirements and thus alleviating angina. Electrical activity through the AV node is responsible for determining heart rate, and this activity is dependent upon calcium influx through L-type calcium channels. By inhibiting these channels and decreasing the influx of calcium, verapamil prolongs the refractory period of the AV node and slows conduction, thereby slowing and controlling the heart rate in patients with arrhythmia. Verapamil's mechanism of action in the treatment of cluster headaches is unclear, but is thought to result from an effect on other calcium channels (e.g. N-, P-, Q-, or T-type). Verapamil is known to interact with other targets, including other calcium channels, potassium channels, and adrenergic receptors.
Calcium antagonists inhibit excitation-contraction coupling in myocardial and smooth muscle by blocking the transmembrane carrier of calcium. This results in decreased myocardial contractility and in vasodilatation. ... /Salt not specified/
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 829
Verapamil has been shown to be neuroprotective in several acute neurotoxicity models due to blockade of calcium entry into neurons. However, the potential use of verapamil to treat chronic neurodegenerative diseases has not been reported. Using rat primary mesencephalic neuron/glia cultures, we report that verapamil significantly inhibited LPS-induced dopaminergic neurotoxicity in both pre- and post-treatment experiments. Reconstituted culture studies revealed that the presence of microglia was essential in verapamil-elicited neuroprotection. Mechanistic studies showed that decreased production of inflammatory mediators from LPS-stimulated microglia underlay neuroprotective property of verapamil. Further studies demonstrated that microglial NADPH oxidase (PHOX), the key superoxide-producing enzyme, but not calcium channel in neurons, is the site of action for the neuroprotective effect of verapamil. This conclusion was supported by the following two observations: 1) Verapamil failed to show protective effect on LPS-induced dopaminergic neurotoxicity in PHOX-deficient (deficient in the catalytic subunit of gp91(phox)) neuron/glia cultures; 2) Ligand binding studies showed that the binding of (3)H-verapamil onto gp91(phox) transfected COS7 cell membranes was higher than the non-transfected control. The calcium channel-independent neuroprotective property of verapamil was further supported by the finding that R(+)-verapamil, a less active form in blocking calcium channel, showed the same potency in neuroprotection, inhibition of pro-inflammatory factors production and binding capacity to gp91(phox) membranes as R(-)-verapamil, the active isomer of calcium channel blocker. In conclusion, our results demonstrate a new indication of verapamil-mediated neuroprotection through a calcium channel-independent pathway and provide a valuable avenue for the development of therapy for inflammation-related neurodegenerative diseases.
PMID:20950631 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3014428 Liu Y et al; Neuropharmacology 60 (2-3): 373-80 (2011)