


1. 9 Beta Arabinofuranosyladenine
2. 9 Beta D Arabinofuranosyladenine
3. 9-beta-arabinofuranosyladenine
4. 9-beta-d-arabinofuranosyladenine
5. Adenine Arabinoside
6. Alpha Ara A
7. Alpha D Arabinofuranosyladenine
8. Alpha-ara A
9. Alpha-d-arabinofuranosyladenine
10. Ara A
11. Ara-a
12. Arabinofuranosyladenine
13. Arabinoside, Adenine
14. Arabinosyladenine
15. Beta Ara A
16. Beta-ara A
17. Vidarabine
18. Vira A
19. Vira-a
20. Viraa
1. 24356-66-9
2. Fa2dm6879k
3. Ci 673
4. Vidarabine (usan)
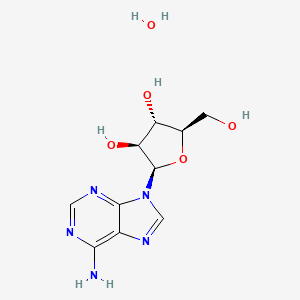
5. (2r,3s,4s,5r)-2-(6-amino-9h-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol Hydrate
6. Vidarabine [usan]
7. (2r,3s,4s,5r)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol;hydrate
8. Vidarabine [jan]
9. Vidarabine (monohydrate)
10. (2r,3s,4s,5r)-2-(6-amino-9h-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol Hydrate
11. Adenine Arabinofuranoside Hydrate
12. 9-beta-d-arabinofuranosyl-9h-purine-6-amine Monohydrate
13. Drg-0058
14. Unii-fa2dm6879k
15. 9-beta-d-arabinofuranosyladenine Monohydrate
16. Vidarabineh2o
17. Vidarabine H2o
18. Adenine, 9-beta-d-arabinofuranosyl-, Monohydrate
19. Vidarabine [usan:usp:inn:ban:jan]
20. 9h-purin-6-amine, 9-beta-d-arabinofuranosyl-, Monohydrate
21. Vira-a (tn)
22. Vidarabine [vandf]
23. Schembl2929
24. Vidarabine [mart.]
25. Chebi:9978
26. Vidarabine [orange Book]
27. Dtxsid10947210
28. Vidarabine [usp Impurity]
29. 9h-purin-6-amine, 9-.beta.-d-arabinofuranosyl-, Monohydrate
30. Vidarabine Monohydrate [mi]
31. Hy-n6666
32. Mfcd00150980
33. S5297
34. Akos025312428
35. Ccg-267319
36. Nsc 757383
37. Cs-0083211
38. 9-beta-d-arabino Furanosyl Adenine Monohydrate
39. Adenine 9-beta-d-arabinofuranoside Monohydrate
40. C07195
41. D00406
42. T72143
43. Vidarabine (200 Mg)g-2939ug/mg(ai)
44. 9-pentofuranosyl-9h-purin-6-amine--water (1/1)
45. J-015462
46. 9-.beta.-d-arabinofuranosyladenine Monohydrate
47. 9h-purin-6-amine,9-b-d-arabinofuranosyl-,hydrate(1:1)
| Molecular Weight | 285.26 g/mol |
|---|---|
| Molecular Formula | C10H15N5O5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 285.10731860 g/mol |
| Monoisotopic Mass | 285.10731860 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 335 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)