


1. Cellblastin
2. Lemblastine
3. Sulfate, Vinblastine
4. Velban
5. Velbe
6. Vinblastin Hexal
7. Vinblastina Lilly
8. Vinblastine
9. Vinblastinsulfat-gry
10. Vincaleukoblastine
1. 143-67-9
2. Velban
3. Velsar
4. Vinblastine (sulfate)
5. Velbe
6. Exal
7. Vlb Monosulfate
8. Alkaban-aq
9. 29060-le
10. Vinblastine Sulphate
11. Vincaleukoblastine,sulfate(1:1)
12. Nsc-49842
13. Mls000863275
14. Vincaleukoblastine, Sulfate (1:1) (salt)
15. N00w22yo2b
16. Belvan, Vlb
17. 29060le
18. Smr000058844
19. Vincaleucoblastine Sulfate
20. Dsstox_cid_27831
21. Dsstox_rid_82594
22. Dsstox_gsid_47853
23. Uniblastin
24. Rozevin Sulfate
25. 29060 Le
26. Cas-18556-44-0
27. Vincaleukoblastine, Sulfate
28. Unii-n00w22yo2b
29. Ccris 2584
30. Vinblastinesulfate
31. Vinblastini Sulfas
32. Chembl378544
33. Ncgc00095285-01
34. Einecs 205-606-0
35. Vinblastine Sulfate [usan:usp:jan]
36. Opera_id_960
37. Ai3-52943
38. Ncgc00181127-01
39. Schembl3549
40. Mls000069550
41. Mls002153253
42. Mls002207069
43. Vinblastine Sulfate [mi]
44. Vinblastine Sulfate [jan]
45. Dtxsid601017133
46. Hms2235e07
47. Hms3403n09
48. Vinblastine Sulfate [iarc]
49. Vinblastine Sulfate [usan]
50. Vinblastine Sulfate [vandf]
51. Vinblastine Sulfate [mart.]
52. Tox21_111498
53. Tox21_112735
54. Ac-821
55. Vinblastine Sulfate [usp-rs]
56. Vinblastine Sulfate [who-dd]
57. Vinblastine Sulfate [who-ip]
58. Akos015960580
59. Tox21_111498_1
60. Tox21_112735_1
61. Cs-1365
62. Ncgc00263548-01
63. Ncgc00344583-01
64. Vinblastine Sulfate [orange Book]
65. Hy-13780
66. Vinblastine Sulfate [ep Monograph]
67. Vinblastini Sulfas [who-ip Latin]
68. Vinblastine Sulfate [usp Monograph]
69. N2255
70. 143v679
71. Sr-01000000155
72. Vinblastine Sulfate Salt, Powder, >=96% (hplc)
73. Sr-01000000155-7
74. Q27077057
75. Vinblastine Sulfate, European Pharmacopoeia (ep) Reference Standard
76. Vinblastine Sulfate, United States Pharmacopeia (usp) Reference Standard
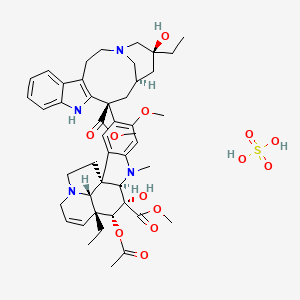
77. Methyl (1r,9r,10s,11r,12r,19r)-11-(acetyloxy)-12-ethyl-4-[(13s,15r,17s)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0?,??.0?,??]nonadeca-4(12),5(10),6,8-tetraen-13-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.0?,?.0?,?.0??,??]nonadeca-2(7),3,5,13-tetraene-10-carboxylate; Sulfuric Acid
78. Methyl (3ar,3a1r,4r,5s,5ar,10br)-4-acetoxy-3a-ethyl-9-((5s,7r,9s)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2h-3,7-methano[1]azacycloundecino[5,4-b]indol-9-yl)-5-hydroxy-8-methoxy-6-methyl-3a,3a1,4,5,5a,6,11,12-octahydro-1h-indolizino[8,1-cd]carbazole-5-carboxylate Sulfate
1. Vincaleukoblastine Sulfate
2. Vinblastine Sulphate
3. Cellblastin
| Molecular Weight | 909.1 g/mol |
|---|---|
| Molecular Formula | C46H60N4O13S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 10 |
| Exact Mass | 908.38775915 g/mol |
| Monoisotopic Mass | 908.38775915 g/mol |
| Topological Polar Surface Area | 237 Ų |
| Heavy Atom Count | 64 |
| Formal Charge | 0 |
| Complexity | 1780 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Vinblastine sulfate |
| PubMed Health | Vinblastine |
| Drug Label | Vinblastine sulfate is the salt of an alkaloid extracted from Vinca rosea Linn., a common flowering herb known as the periwinkle (more properly known as Catharanthus roseus G. Don). Previously, the generic name was vincaleukoblastine, abbreviated VLB... |
| Active Ingredient | Vinblastine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/vial; 1mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Vinblastine sulfate |
| PubMed Health | Vinblastine |
| Drug Label | Vinblastine sulfate is the salt of an alkaloid extracted from Vinca rosea Linn., a common flowering herb known as the periwinkle (more properly known as Catharanthus roseus G. Don). Previously, the generic name was vincaleukoblastine, abbreviated VLB... |
| Active Ingredient | Vinblastine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/vial; 1mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)

