


1. Compound 112531
2. Desacetylvinblastine Amide
3. Eldisine
4. Enison
5. Nsc 245467
6. Nsc-245467
7. Nsc245467
8. Sulfate, Vindesine
9. Vindesin
10. Vindesine
1. 59917-39-4
2. Vindesine Sulfate Salt
3. Fildesin (tn)
4. Vindesinesulfatesalt
5. Eldesine
6. Eldisine
7. Desacetylvinblastine Amide Sulfate
8. Chebi:32295
9. Dava
10. Vindesine (sulfate)
11. 3-carbamoyl-4-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine Sulfate (1:1) (salt)
12. 3-carbamoyl-o(4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine Sulfate
13. Fildesi
14. Vindesine Sulfate- Bio-x
15. Desacetyl Vinblastine Amide
16. Schembl4285
17. Dsstox_cid_29000
18. Dsstox_rid_83265
19. Dsstox_gsid_49074
20. Vindesine Sulfate(rg)
21. Mls001424270
22. Vindesine Sulfate Salt Hydrate
23. Vdsdesacetyl Vinblastine Amide
24. Vindesine Sulfate (jan/usan)
25. Chembl2105882
26. Dtxsid0049074
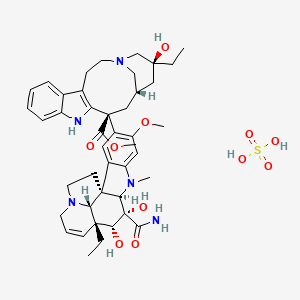
27. C43h57n5o11s
28. Hms2052b03
29. N-a-boc-d-2,4-diaminobutyricacid
30. Tox21_113632
31. S2440
32. Akos015895863
33. Ccg-101151
34. Nc00401
35. Ac-24198
36. Bv164524
37. Smr000469153
38. Cas-59917-39-4
39. Hy-129071
40. Cs-0103448
41. D01769
42. 917v394
43. Q-100693
| Molecular Weight | 852.0 g/mol |
|---|---|
| Molecular Formula | C43H57N5O11S |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 7 |
| Exact Mass | 851.37752882 g/mol |
| Monoisotopic Mass | 851.37752882 g/mol |
| Topological Polar Surface Area | 248 Ų |
| Heavy Atom Count | 60 |
| Formal Charge | 0 |
| Complexity | 1650 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)