
1. Pyostacine
2. Rp 7293
3. Rp7293
1. Virginiamycin
2. Stafac
3. 270076-60-3
4. Pristinamycine
5. Staphylomycin
6. 11006-76-1
7. Eskalin V
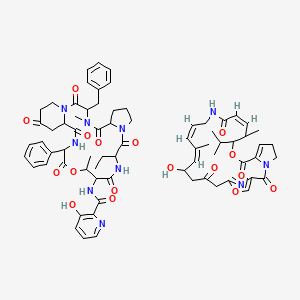
8. N-(3-benzyl-12-ethyl-4,16-dimethyl-2,5,11,14,18,21,24-heptaoxo-19-phenyl-17-oxa-1,4,10,13,20-pentazatricyclo[20.4.0.06,10]hexacosan-15-yl)-3-hydroxypyridine-2-carboxamide;(12z,17z,19z)-21-hydroxy-11,19-dimethyl-10-propan-2-yl-9,26-dioxa-3,15,28-triazatricyclo[23.2.1.03,7]octacosa-1(27),6,12,17,19,25(28)-hexaene-2,8,14,23-tetrone
9. Stafytracine
10. Virgimycine
11. Starfac
12. Stajac 22
13. Schembl132821
14. Nsc246121
15. Nsc-246121
| Molecular Weight | 1349.5 g/mol |
|---|---|
| Molecular Formula | C71H84N10O17 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 7 |
| Exact Mass | 1348.60159125 g/mol |
| Monoisotopic Mass | 1348.60159125 g/mol |
| Topological Polar Surface Area | 364 Ų |
| Heavy Atom Count | 98 |
| Formal Charge | 0 |
| Complexity | 2640 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 10 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
MEDICATON (VET): Necrotic enteritis is an acute enterotoxemia. The clinical illness is usually very short and often the only signs are a sudden increase in mortality. The disease primarily affects broiler chickens (2-5 wk old) and turkeys (7-12 wk old) raised on litter but can also affect commercial layer pullets raised in cages. ... Because Clostridium perfringens is nearly ubiquitous, it is important to prevent changes in the intestinal microflora that would promote its growth. This can be accomplished by adding antibiotics in the feed such as virginiamycin ... .
Kahn, C.M (ed.).; The Merck Veterinary Manual 10th Edition. Merck & Co. Whitehouse Station NJ. 2010, p. 2406
MEDICATION (VET): Swine dysentery is a common, mucohemorrhagic diarrheal disease of pigs that affects the large intestine. ... Therapeutic use of antibacterials is effective if started early. ... Virginiamycin /is/ commonly used.
Kahn, C.M (ed.).; The Merck Veterinary Manual 10th Edition. Merck & Co. Whitehouse Station NJ. 2010, p. 278
MEDICATION (VET): For use in cattle fed in confinement for slaughter: Improved feed efficiency. Reduction of incidence of liver abscesses. Increased rate of weight gain.
US Natl Inst Health; DailyMed. Current Medication Information for V-MAX (virginiamycin) powder (April 2010). Available from, as of July 19, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=de8f877b-06fe-41bc-a409-d4e5546cb5da
MEDICATION (VET): Efficacy of virginiamycin (22 mg/kg) in combination with no drug, amprolium, carbarsone, halofuginone, or monensin, was studied. Male & female turkeys were raised to market age in five experiments conducted from 1983 to 1987. Body weights & feed:gain responses to virginiamycin for males & females were positive & significant (P less than .05). Virginiamycin resulted in mean 5.2 & 6.3% body weight responses & 3.3 & 2.2% feed:gain responses for males at 19 or 20 wk of age & for females at 16 or 17 wk of age, respectively. Mortality rates were low in all studies, & were not influenced by virginiamycin. In a processing study, virginiamycin in combination with halofuginone did not affect shrinkage, yield, or market grade. Feed was utilized by males & females 3.9 & 3.0%, respectively, more efficiently than expected with dietary virginiamycin, compared with results predicted by a simulation modeling technique. Profitability was considerably greater with dietary virginiamycin using actual data than with simulated feed consumption data.
PMID:1908578 Waibel PE, et al; Poult Sci 70 (4): 837-847 (1991)
VET: The effects of dietary supplements of virginiamycin on the behavior and physiology of 17 thoroughbred geldings (five cribbers, six weavers and six control horses) were compared with the effects of a placebo over a period of 16 weeks. Virginiamycin had no effect on the horses' stereotypic behavior, but it reduced their explorative behavior, possibly owing to a reduction in feeding motivation. Virginiamycin increased the water intake of the cribbers and decreased the water intake of the control horses, but it was not possible to eliminate possible confounding factors for this effect. Virginiamycin had no other significant effects on the behavior or physiology of the horses, and had no effect on the digestibility of their diets.
PMID:18836155 Freire R et al; Vet Rec 163 (14): 413-7 (2008)
VET: The effect of a peptolide antibiotic virginiamycin on the growth, rumen & blood parameters was followed in 8 milk-fed calves, 4 weeks old initially. Calves were individually housed in metabolic cages. The experiment was ended at the age of 16 weeks. Virginiamycin was supplied at 80 mg per head per day. Calves receiving virginiamycin gained 5.1% more than control calves. Feed intake per 1 kg of body weight gain was higher in control calves. Virginiamycin significantly increased molar percentage of propionate & decreased molar acetate: propionate ratio in rumen fluid. Serum iron, hematocrit & hemoglobin were significantly increased in the treated group in the last period of the trial. Virginiamycin lowered serum protein & urea & tended to decr activity of aminotransferases.
PMID:8215884 Skrivanova V, Marounek M; Arch Tierernahr 44 (1): 41-46 (1993)
VET: Morphologic studies were carried out on the liver, kidneys, and small intestine of broiler birds that had been given antibiotics with the feed (virginiamycin, 20.0 ppm, flavomycin, 4.8 ppm, and avotan, 10.0 ppm) in the course of 49 days, kept with a group of controls. The liver of the test birds showed protein and fatty dystrophy, and the kidneys--protein dystrophy. The small intestine showed thinning of the wall and increase in the villi intestinales length. The manifestation of the morphologic changes depended on the amount of the antibiotic taken in. Those of the birds that were offered flavomycin had well manifested lesions, while in birds that were given virginiamycin and avotan only the lesions were more slightly expressed.
PMID:3113045 Veselinova A et al; Vet Med Nauki 24 (1): 9-14 (1987)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX10 - Virginiamycin
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FG - Streptogramins
J01FG01 - Pristinamycin
The results of residue determinations of the growth promotors carbadox, tylosin, & virginiamycin in kidney, liver, & muscle from pigs in feeding experiments are described as well as the analytical methods used. Residues of the carbadox metabolite quinoxaline-2-carboxylic acid were found in liver from pigs fed 20 mg/kg in the diet with a withdrawal time of 30 days. No residues were detected in muscle with zero withdrawal time. The limit of determination was 0.01 mg/kg for both tissues. No residues of virginiamycin & tylosin were found in pigs fed 50 & 40 mg/kg, respectively, in the diet, even with zero withdrawal time. Residues of tylosin of 0.06 mg/kg & below were detected in liver & kidney from pigs fed 200 or 400 mg/kg & slaughtered within 3 h after the last feeding.
PMID:3148611 Lauridsen MG, et al; J Assoc Off Anal Chem 71 (5): 921-925 (1988)
Reduction of virginiamycin S with sodium borohydride produces allo- & normal-dihydro-virginiamycin S. Reduction of the tosylhydrazone of virginiamycin S with sodium cyanoborohydride affords deoxyvirginiamycin S. These compounds are less active than virginiamycin S. Like virginiamycin S they enhance the activity of virginiamycin M1.
PMID:403168 Janssen G, et al; J Antibiot (Tokyo) 30 (2): 141-145 (1977)
Virginiae butanolides (VBs), which are among the butyrolactone autoregulators of Streptomyces species, act as a primary signal in Streptomyces virginiae to trigger virginiamycin biosynthesis & possess a specific binding protein, BarA. To clarify the in vivo function of BarA in the VB-mediated signal pathway that leads to virginiamycin biosynthesis, two barA mutant strains (strains NH1 & NH2) were created by homologous recombination. In strain NH1, an internal 99-bp EcoT14I fragment of barA was deleted, resulting in an in-frame deletion of 33 amino acid residues, including the second helix of the probable helix-turn-helix DNA-binding motif. With the same growth rate as wild-type S. virginiae on both solid & liquid media, strain NH1 showed no apparent changes in its morphological behavior, indicating that the VB-BarA pathway does not participate in morphological control in S. virginiae. In contrast, virginiamycin production started 6 hr earlier in strain NH1 than in the wild-type strain, demonstrating for the first time that BarA is actively engaged in the control of virginiamycin production & implying that BarA acts as a repressor in virginiamycin biosynthesis. In strain NH2, an internal EcoNI-SmaI fragment of barA was replaced with a divergently oriented neomycin resistance gene cassette, resulting in the C-terminally truncated BarA retaining the intact helix-turn-helix motif. In strain NH2 & in a plasmid-integrated strain containing both intact & mutated barA genes, virginiamycin production was abolished irrespective of the presence of VB, suggesting that the mutated BarA retaining the intact DNA-binding motif was dominant over the wild-type BarA. These results further support the hypothesis that BarA works as a repressor in virginiamycin production & suggests that the helix-turn-helix motif is essential to its function. In strain NH1, VB production was also abolished, thus indicating that BarA is a pleiotropic regulatory protein controlling not only virginiamycin production but also autoregulator biosynthesis.
PMID:9642182 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC107284 Nakano H, et al; J Bacteriol 180 (13): 3317-3322 (1998)
Previous findings suggest the location of the central loop of domain V of 23S rRNA within the peptidyltransferase domain of ribosomes. This enzymatic activity is inhibited by some antibiotics, including type A (virginiamycin M or VM) & type B (virginiamycin S or VS) synergimycins, antibiotics endowed with a synergistic action in vivo. In the present work, the ability of VM & VS to modify the accessibility of 23S rRNA bases within ribosomes to chemical reagents has been explored. VM afforded a protection of rRNA bases A2037, A2042, G2049 & C2050. Moreover, when ribosomes were incubated with the two virginiamycin components, the base A2062, which was protected by VS alone, became accessible to dimethyl sulphate (DMS). Modified reactivity to chemical reagents of different rRNA bases located either in the central loop of domain V or in its proximity furnishes experimental evidence for conformational ribosome alterations induced by VM binding
PMID:7971275 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC308478 Vannuffel P, et al; Nucleic Acids Res 22 (21): 4449-4453 (1994)