


1. Chocola A
2. Retinol Palmitate
3. Retinyl Palmitate
1. Retinyl Palmitate
2. 79-81-2
3. Retinol Palmitate
4. Retinol, Hexadecanoate
5. All-trans-retinyl Palmitate
6. Arovit
7. Optovit-a
8. Retinyl Hexadecanoate
9. Aquapalm
10. Dispatabs Tabs
11. Vitazyme A
12. Optovit A
13. Axerophthol Palmitate
14. Trans-retinol Palmitate
15. Trans-retinyl Palmitate
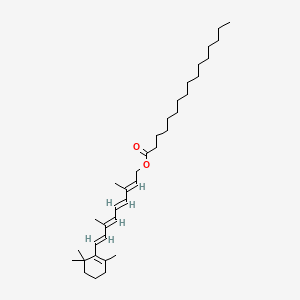
16. [(2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenyl] Hexadecanoate
17. Lutavit A 500 Plus
18. All-trans-retinol Palmitate
19. Arovit (roche)
20. All-trans-vitamin A Palmitate
21. Aquasol A (tn)
22. Retinyl (vitamin A) Palmitate
23. Retinol Palmitate [jan]
24. O~15~-hexadecanoylretinol
25. Nsc-758478
26. 1d1k0n0vvc
27. Retinol, Palmitate, All-trans
28. Ester Found In Fish Liver Oils
29. Retinol Palmitate (6ci,7ci)
30. Chebi:17616
31. Palmitic Acid, Ester With Retinol
32. Retinol, Palmitate, All-trans- (8ci)
33. Ncgc00095056-03
34. Dsstox_cid_1241
35. Dsstox_rid_76033
36. Dsstox_gsid_21241
37. (2e,4e,6e,8e)-hexadecanoic Acid 3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2, 4,6,8,tetraenyl Ester
38. Vitamin A Solubilized
39. Cas-79-81-2
40. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-yl Hexadecanoate
41. Smr000112463
42. Ccris 3280
43. Retinol, Palmitate, All-trans-
44. Einecs 201-228-5
45. Unii-1d1k0n0vvc
46. Brn 1917366
47. Retinylpalmitate
48. Retinol-palmitate
49. Retinyl-palmitate
50. Retinol, All-trans-, Palmitate
51. Palmitic Acid Retinol
52. Retinyl Palmitic Acid
53. Vitamin- A Palmitate
54. Retinyl Hexadecanoic Acid
55. Spectrum5_001201
56. All-(e)-retinol Palmitate
57. Bmse000501
58. Ec 201-228-5
59. Retinol Palmitate (jp17)
60. Chembl1675
61. Schembl41649
62. Mls001332437
63. Mls001332438
64. Spectrum1503604
65. All-trans-retinyl Hexadecanoate
66. Dtxsid1021241
67. Retinyl Palmitate [inci]
68. Hms500m11
69. Retinyl Palmitate [vandf]
70. Vitamin A Palmitate [mi]
71. Hms1922e10
72. Hms2093g13
73. Hms2268c06
74. Pharmakon1600-01503604
75. Retinol Palmitate [who-dd]
76. Retinyl Palmitate [usp-rs]
77. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-yl Palmitate
78. Amy13840
79. Hy-b1384
80. Vae 16:0
81. Vitamin A Palmitate [vandf]
82. Zinc8214494
83. Tox21_113452
84. Tox21_303008
85. Ccg-39342
86. Lmpr01090013
87. Mfcd00019414
88. Nsc758478
89. S4126
90. Retinol, O~15~-(1-oxohexadecyl)-
91. Akos015918435
92. Nsc 758478
93. Vitamin A Palmitate, 1.7 M.i.u./g
94. Idi1_000249
95. Ncgc00095056-01
96. Ncgc00095056-02
97. Ncgc00256427-01
98. Ac-20001
99. Vitamin A Palmitate [orange Book]
100. Sbi-0051830.p002
101. All-(e)-retinol Palmitate [who-ip]
102. Cs-0013116
103. C02588
104. C76552
105. D00164
106. Ab00052360_04
107. A839762
108. Sr-05000001910
109. Vitamin A Palmitate (solubilized) [vandf]
110. Q7316807
111. Sr-05000001910-1
112. Retinyl Palmitate (vitamin A Palmitate; Retinol Palmitate)
113. Retinyl Palmitate, Potency: >=1,700,000 Usp Units Per G
114. Vitamin A (as Palmitate & Beta Carotene) [vandf]
115. Retinyl Palmitate, Type Iv, ~1,800,000 Usp Units/g, Oil
116. Retinyl Palmitate, United States Pharmacopeia (usp) Reference Standard
117. [3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenyl] Hexadecanoate
118. 3,7-dimethyl-9-(2,6,6,-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol Palmitate
119. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-en-1-yl)-2,4,6,8-nonateetraen-1-yl-palmitate
120. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetraenyl Palmitate
121. 110067-62-4
122. Hexadecanoic Acid [(2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenyl] Ester
123. Retinyl Palmitate (vitamin A Palmitate), Pharmaceutical Secondary Standard; Certified Reference Material
124. Retinyl Palmitate Solution, 100 Mug/ml (ethanol With 0.1% (w/v) Bht), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 524.9 g/mol |
|---|---|
| Molecular Formula | C36H60O2 |
| XLogP3 | 13.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 21 |
| Exact Mass | 524.45933115 g/mol |
| Monoisotopic Mass | 524.45933115 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 803 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticarcinogenic Agents
Agents that reduce the frequency or rate of spontaneous or induced tumors independently of the mechanism involved. (See all compounds classified as Anticarcinogenic Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
