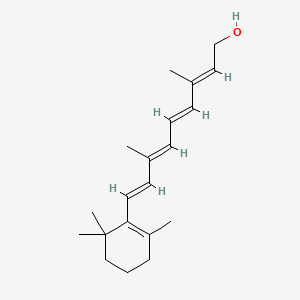

1. 11-cis-retinol
2. 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol, (all-e)-isomer
3. All Trans Retinol
4. All-trans-retinol
5. Aquasol A
6. Vitamin A
7. Vitamin A1
1. Vitamin A
2. All-trans-retinol
3. 68-26-8
4. Vitamin A1
5. Alphalin
6. Axerophthol
7. Afaxin
8. Vitamin A Alcohol
9. Oleovitamin A
10. Chocola A
11. Alphasterol
12. Apostavit
13. Aquasynth
14. Biosterol
15. Epiteliol
16. Ophthalamin
17. Agiolan
18. Agoncal
19. Anatola
20. Myvpack
21. Prepalin
22. Testavol
23. Veroftal
24. Aoral
25. Apexol
26. Avibon
27. Avitol
28. Axerol
29. Dofsol
30. Trans-retinol
31. Vaflol
32. Vitpex
33. Vogan
34. Disatabs Tabs
35. Lard Factor
36. All-trans-retinyl Alcohol
37. Bentavit A
38. Dohyfral A
39. Alcovit A
40. Anatola A
41. Vogan-neu
42. A-mulsal
43. Plivit A
44. Vi-alpha
45. A-vitan
46. All-trans Retinol
47. Atars
48. Vafol
49. All-trans-vitamin A Alcohol
50. Retrovitamin A
51. Homagenets Aoral
52. A-sol
53. Hi-a-vita
54. Sehkraft A
55. Vitamin A1 Alcohol
56. All-trans-vitamin A
57. A-vi-pel
58. Acon
59. Atav
60. Vi-dom-a
61. 11103-57-4
62. Super A
63. Anti-infective Vitamin
64. Solu-a
65. Nio-a-let
66. Vio-a
67. Antixerophthalmic Vitamin
68. Del-vi-a
69. Vitavel A
70. Thalasphere
71. Beta-retinol
72. Trans-vitamin A Alcohol
73. Vitamin A Alcohol, All-trans-
74. Vitamin A1, All-trans-
75. Retinol, All Trans-
76. All-trans-vitamin A1
77. Vitamin A Oil
78. .beta.-retinol
79. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol
80. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraen-1-ol
81. Vitamin A1 Alcohol, All-trans-
82. (all-e)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol
83. Chembl986
84. Nsc-122759
85. G2sh0xkk91
86. 2,4,6,8-nonatetraen-1-ol, 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (all-e)-
87. Axerophtholum
88. Chebi:17336
89. Wachstumsvitamin
90. All-trans-13,14-dihydro Retinol
91. Vitaminum A
92. Vitamine A
93. Vitavel-a
94. Vitamina
95. Retinolo [dcit]
96. Ncgc00017343-07
97. Cylasphere
98. Retinolo
99. Retinolum
100. Retinol [inn:ban]
101. Dsstox_cid_3556
102. Hydrovit A
103. Retinolum [inn-latin]
104. 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol
105. Dsstox_rid_77080
106. Dsstox_gsid_23556
107. Ro-a-vit
108. Trol
109. Vitamin A Alcohol (van)
110. Antixerophthalmisches Vitamin
111. Aquasol A Parenteral
112. Vi-alpha; Vi-alpha
113. Rovimix A 500
114. 9-cis Retinol
115. Vitamin A1 Alcohol, All Trans
116. Retinol Solution
117. Cas-68-26-8
118. Smr000112036
119. Retinol (vit A)
120. [11,12-3h]-retinol
121. 9-cis,13-cis-retinol
122. Vitamin A (usp)
123. Ccris 5444
124. Hsdb 815
125. Vitamin A [natural]
126. Sr-01000763813
127. Retin-11,12-t2-ol (9ci)
128. Einecs 200-683-7
129. Mfcd00001552
130. Unii-g2sh0xkk91
131. Nsc 122759
132. Brn 0403040
133. Unii-81g40h8b0t
134. Tegosphere Vita
135. .alpha.sterol
136. B-retinol
137. .alpha.lin
138. Retinyl A
139. Trans-retinol Acid (vitamin A)
140. 1rbp
141. Vi-.alpha.
142. Einecs 234-328-2
143. 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclchexen-1-yl)-2,4,6,8-nonatetraen-1-ol
144. (9z)-retinol
145. 1gx8
146. Retinol-(cellular-retinol-binding-protein)
147. Retinol [hsdb]
148. Retinol [inci]
149. Retinol [inn]
150. Spectrum5_000993
151. Spectrum5_001997
152. Vitamin A [mi]
153. Retinol [who-dd]
154. Retinol, 95%, Synthetic
155. Ec 200-683-7
156. All-trans Vitamin A Alcohol
157. Schembl3112
158. 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonate-traen-1-ol
159. 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol, (all-e)-
160. Alcohol 9,13-dimethyl-7-(1,1,5-trimethyl-6-cyclohexen-5-yl)-7,9,11,13-nonatetraen-15-ol
161. Bidd:pxr0102
162. 4-06-00-04133 (beilstein Handbook Reference)
163. Mls001066379
164. Mls001074751
165. Mls006010008
166. Retinol, All-trans- (8ci)
167. Spectrum1501203
168. Gtpl4053
169. Dtxsid3023556
170. Hms501i08
171. 81g40h8b0t
172. Hms1921b04
173. Hms2092l13
174. Hms2270c05
175. Pharmakon1600-01501203
176. Bcp06593
177. Hy-b1342
178. Zinc3831417
179. Tox21_110818
180. Tox21_202441
181. Tox21_300287
182. Bdbm50092056
183. Ccg-38864
184. Lmpr01090001
185. Nsc122759
186. Nsc758150
187. S5592
188. Akos015902578
189. Db00162
190. Nsc-758150
191. Sdccgmls-0066724.p001
192. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetr Aen-1-ol
193. Idi1_000486
194. Retinol Solution, >=95.0% (hplc)
195. Smp2_000102
196. Ncgc00017343-02
197. Ncgc00017343-03
198. Ncgc00017343-04
199. Ncgc00017343-05
200. Ncgc00017343-06
201. Ncgc00017343-08
202. Ncgc00017343-09
203. Ncgc00017343-11
204. Ncgc00091784-01
205. Ncgc00091784-02
206. Ncgc00091784-03
207. Ncgc00091784-04
208. Ncgc00091784-05
209. Ncgc00091784-06
210. Ncgc00254024-01
211. Ncgc00259990-01
212. Ac-11701
213. Bs-17906
214. Sbi-0051690.p002
215. Cs-0013091
216. C00473
217. C17276
218. D06543
219. Ab00052248_05
220. 103v574
221. A836068
222. Q424976
223. Retinol, >=95.0% (hplc), ~2700 U/mg
224. Retinol, Synthetic, >=95% (hplc), Crystalline
225. J-014834
226. J-017515
227. Q-201926
228. Sr-01000763813-2
229. Sr-01000763813-4
230. W-104683
231. Brd-k22429181-001-06-8
232. Brd-k64634304-001-01-5
233. Wln: L6utj A1 B1u1y1&u2u1y1&u2q C1 C1
234. Retinol, Bioxtra, >=97.5% (hplc), ~3100 U/mg
235. 3,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol
236. 2,6,8-nonatetraen-1-ol, 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (all-e)-
237. 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol, All (e)-
238. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)-1-nona-2,4,6,8-tetraenol
239. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraen-1-ol
240. (2e,4e,6e,8e)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetraen-1-ol
241. (2z,4z,6z,8z)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetren-1-ol
242. Retinol Solution, 100 Mug/ml +/- 25% (refer To Coa) (ethanol With 0.1% (w/v) Bht), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 286.5 g/mol |
|---|---|
| Molecular Formula | C20H30O |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 5 |
| Exact Mass | 286.229665576 g/mol |
| Monoisotopic Mass | 286.229665576 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 496 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vitamin A is indicated only for prevention or treatment of vitamin A deficiency states. Vitamin A deficiency may occur as a result of inadequate nutrition or intestinal malabsorption but does not occur in healthy individual receiving an adequate balanced diet. For prophylaxis of vitamin A deficiency, dietary improvement, rather than supplementation, is advisable. For treatment of vitamin A deficiency, supplementation is preferred. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
Recommended intakes may be increased and/or supplementation may be necessary in infants receiving unfortified formula or in individuals with the following conditions (based on documented vitamin A deficiency): Diarrhea; gastrectomy; hyperthyroidism; infections, chronic; intestinal diseases: celiac, diarrhea, topical sprue, regional enteritis; malabsorption syndromes associated with pancreatic insufficiency: pancreatic disease, cystic fibrosis; measles; protein deficiency, severe, stress, prolonged; xerophthalmia. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
Some unusual diets (e.g., reducing diets that drastically restrict food selection, especially the fat-containing foods) may not supply minimum daily recommended intakes of vitamin A. Supplementation is necessary in patients receiving total parenteral nutrition (TPN) or undergoing rapid weight loss or in those with malnutrition, because of inadequate dietary intake.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
Recommended intakes for most vitamins and minerals are increased during pregnancy. Many physicians recommend that pregnant women receive multivitamin and mineral supplements, especially those pregnant women who do not consume an adequate diet and those in high-risk categories (i.e., women carrying more than one fetus, heavy cigarette smokers, and alcohol and drug abusers). Taking excessive amounts of a multivitamin and mineral supplement may be harmful to the mother and/or fetus and should be avoided.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
For more Therapeutic Uses (Complete) data for VITAMIN A (7 total), please visit the HSDB record page.
Pregnancy risk category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweights any possible benefit to the patient./ /Parenteral vitamin A/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2842
Doses of vitamin A that do not exceed the physiologic requirement are usually nontoxic.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 3490
There are insufficient data to show that vitamin A may reduce the occurrence of certain types of cancer.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
... Vitamin A has not been proven effective for treatment of renal calculi, hyperthyroidism, anemia, degenerative conditions of the nervous system, sunburn, lung diseases, deafness, osteoarthritis, inflammatory bowel disease, or psoriasis.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
For more Drug Warnings (Complete) data for VITAMIN A (9 total), please visit the HSDB record page.
For the treatment of vitamin A deficiency.
Vitamin A is effective for the treatment of Vitamin A deficiency. Vitamin A refers to a group of fat-soluble substances that are structurally related to and possess the biological activity of the parent substance of the group called all-trans retinol or retinol. Vitamin A plays vital roles in vision, epithelial differentiation, growth, reproduction, pattern formation during embryogenesis, bone development, hematopoiesis and brain development. It is also important for the maintenance of the proper functioning of the immune system.
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11C - Vitamin a and d, incl. combinations of the two
A11CA - Vitamin a, plain
A11CA01 - Retinol (vit A)
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AD - Retinoids for topical use in acne
D10AD02 - Retinol
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AX - Other nasal preparations
R01AX02 - Retinol
S - Sensory organs
S01 - Ophthalmologicals
S01X - Other ophthalmologicals
S01XA - Other ophthalmologicals
S01XA02 - Retinol
Absorption
Readily absorbed from the normal gastrointestinal tract
Vitamin A is distributed into breast milk ... .
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2842
Less than 5% of circulating vitamin A is bound to lipoproteins in blood (normal), but may be up to 65% when hepatic stores are saturated because of excessive intake. The amount of vitamin A bound to lipoproteins may be increased in hyperlipoproteinemia. When released from liver, vitamin A is bound to retinol-binding protein (RBP). Most vitamin A circulates in the form of retinol bound to RBP.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
Storage: Hepatic (approximately 2 years' adult requirements), with small amounts stored in kidney and lung tissues. Zinc is required for mobilization of vitamin A reserves in the liver.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2841
More than 90% of the intake of preformed vitamin A is in the form of retinol esters, usually as retinyl palmitate. ... When a large excess is ingested, some of the vitamin escapes in the feces. ... Absorption ... is related to that of lipid and is enhanced by bile. ... Aqueous dispersions ... are absorbed more rapidly than are oily solution.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1779
For more Absorption, Distribution and Excretion (Complete) data for VITAMIN A (9 total), please visit the HSDB record page.
Hepatic. Retinol is conjugated with glucuronic acid; the B-glucuronide undergoes enterohepatic circulation and oxidation to retinol and retinoic acid. Retinoic acid undergoes decarboxylation and conjugation with glucuronic acid.
Retinol is converted to retinyl phosphate in epithelial tissues, and this intermediate is in turn metabolized to mannosylretinylphosphate in a reaction that is catalyzed by a microsomal enzyme and requires guanosine diphosphomannose as a glycosyl donor. ... /the vitamin A/ mediates transfer of mannose to specific glycoproteins.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1777
Retinol is in part conjugated to form a beta-glucuronide, which undergoes enterohepatic circulation and is oxidized to retinal and retinoic acid.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1781
Within the retina, all-trans-retinol is oxidized to retinal by alcohol dehydrogenases, and is then Isomerized to the 11-cis-isomer which combines with opsin in the rod to yield rhodopsin, and with different opsins in human cones to yield three different iodopsin pigments.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 254
Retinoic acid (RA) is the bioactive metabolite of vitamin A (retinol) which acts on cells to establish or change the pattern of gene activity. Retinol is converted to RA by the action of two types of enzyme, retinol dehydrogenases and retinal dehydrogenases. In the nucleus RA acts as a ligand to activate two families of transcription factors, the RA receptors (RAR) and the retinoid X receptors (RXR) which heterodimerize and bind to the upstream sequences of RA-responsive genes.
PMID:10828175 Maden M; Proc Nutr Soc 59 (1): 65-73 (2000)
Retinol has known human metabolites that include 4-Hydroxyretinol and retinal.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
1.9 hours
Hepatic reserves of vitamin A decrease with a half-life of about 50 days in animals ...
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1589
Vision:Vitamin A (all-trans retinol) is converted in the retina to the 11-cis-isomer of retinaldehyde or 11-cis-retinal. 11-cis-retinal functions in the retina in the transduction of light into the neural signals necessary for vision. 11-cis-retinal, while attached to opsin in rhodopsin is isomerized to all-trans-retinal by light. This is the event that triggers the nerve impulse to the brain which allows for the perception of light. All-trans-retinal is then released from opsin and reduced to all-trans-retinol. All-trans-retinol is isomerized to 11-cis-retinol in the dark, and then oxidized to 11-cis-retinal. 11-cis-retinal recombines with opsin to re-form rhodopsin. Night blindness or defective vision at low illumination results from a failure to re-synthesize 11-cis retinal rapidly.
Epithelial differentiation: The role of Vitamin A in epithelial differentiation, as well as in other physiological processes, involves the binding of Vitamin A to two families of nuclear retinoid receptors (retinoic acid receptors, RARs; and retinoid-X receptors, RXRs). These receptors function as ligand-activated transcription factors that modulate gene transcription. When there is not enough Vitamin A to bind these receptors, natural cell differentiation and growth are interrupted.
Topical vitamin A can reverse the impairment of wound healing seen in patients receiving corticosteroids, perhaps by restoring the normal inflammatory reaction in the wound. The possibility has been suggested that systemic vitamin A could inhibit the anti-inflammatory effect of systemic corticosteroids.
Hansten P.D. Drug Interactions. 5th ed. Philadelphia: Lea and Febiger, 1985., p. 322
Retinol arrested proliferation of cultured neuroblastoma cells at concentrations of 50 um. A correlation existed between inhibition of growth and inhibition of ornithine decarboxylase in both neuroblastoma cells and glioma cells with retinol.
PMID:6770209 Chapman S; LIFE SCI 26 (16): 1359 (1980)
In rats exptl-hypervitaminosis A has been shown ... to produce severe damage of the retina, mainly in the pigment epithelium according to electron microscopy. Alcohol dehydrogenase activity was shown to disappear in the pigment epithelium and visual cells ... .
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 9795
/The authors/ have shown that in an experimental cell culture system consisting of carcinogen-treated 10T1/2 cells, both retinoids and all dietary carotenoids examined can reversibly inhibit neoplastic transformation in the post-initiation phase of carcinogenesis. This activity strongly correlates with their ability to increase gap junctional intercellular communication by up-regulating the expression of the gene CX43 (connexin43). Connexins comprise the structural unit of gap junctions, organelles which allow direct transfer of signals, nutrients and waste products between contacting cells. CX43 is the most widely expressed member of the gap junction family of genes, and we have demonstrated that its expression is strongly down-regulated in human cancers and in several premalignant conditions. When several human tumour cell lines were genetically engineered to conditionally express CX43 under the influence of a tetracycline promoter, their neoplastic phenotype was strongly attenuated. Specifically, induced cells were inhibited from growing in an anchorage-independent manner and, additionally, growth as xenografts in immunocompromised animals was also strongly attenuated. Growth inhibition in suspension was associated both with increased G(1) cell-cycle arrest and with increased apoptosis. /The authors/ propose a model whereby junctional communication allows the transfer of growth inhibitory signals from normal to neoplastic cells and that retinoids and carotenoids, by increasing signal transfer, act to prevent cancer.
PMID:15506943 Bertram JS; Biochem Soc Trans 32 (Pt 6) :985-9 (2004)