
1. 18f Suberoylanilide Hydroxamic Acid
2. 18f-saha
3. 18f-suberoylanilide Hydroxamic Acid
4. M344
5. Mk 0683
6. Mk-0683
7. Mk0683
8. N Hydroxy N' Phenyloctanediamide
9. N-hydroxy-n'-phenyloctanediamide
10. N1 Hydroxy N8 Phenyloctanediamide
11. N1-hydroxy-n8-phenyloctanediamide
12. Nhnpoda
13. Suberanilohydroxamic Acid
14. Suberoyl Anilide Hydroxamic Acid
15. Suberoylanilide Hydroxamic Acid
16. Zolinza
1. 149647-78-9
2. Saha
3. Suberoylanilide Hydroxamic Acid
4. Zolinza
5. N-hydroxy-n'-phenyloctanediamide
6. N1-hydroxy-n8-phenyloctanediamide
7. Suberanilohydroxamic Acid
8. Mk-0683
9. Mk0683
10. Octanediamide, N-hydroxy-n'-phenyl-
11. N'-hydroxy-n-phenyloctanediamide
12. Vorinostat (saha, Mk0683)
13. Octanedioic Acid Hydroxyamide Phenylamide
14. Ccris 8456
15. N-hyrdroxy-n'-phenyloctanediamide
16. Nsc-701852
17. Vorinostat (saha)
18. Shh
19. Chembl98
20. Mfcd00945317
21. Nsc-748799
22. Nsc-759852
23. 58ifb293ji
24. Chebi:45716
25. N1-hydroxy-n8-phenyl-octanediamide
26. Win64652
27. Nsc701852
28. Saha Cpd
29. Ncgc00168085-01
30. Ncgc00168085-02
31. Vorinostat [usan]
32. Zolinza (tn) (merck)
33. Saha-d5
34. Dsstox_cid_21133
35. Dsstox_rid_79632
36. Dsstox_gsid_41133
37. N-hydroxy-n'-phenyl-octane-1,8-diotic Acid Diamide
38. Vorinostat Msd
39. Smr000486344
40. Zolinza (tn)
41. Cas-149647-78-9
42. Sr-05000000373
43. Vorinostat (jan/usan)
44. Vorinostat [usan:inn]
45. Mk 0683
46. Vorinostatum
47. Unii-58ifb293ji
48. Suberoylanilidehydroxamic Acid
49. Ski390
50. Hsdb 7930
51. 4lxz
52. Vorinostat(saha)
53. Zolinza; Saha
54. Vorinostat - Saha
55. Saha, Suberoylanilide Hydroxamic Acid
56. 1zz1
57. Vorinostat [mi]
58. Sw-064652
59. Vorinostat [inn]
60. Vorinostat [jan]
61. 8-(hydroxyamino)-8-oxo-n-phenyl-octanamide
62. Vorinostat [vandf]
63. Cid_5311
64. Schembl9018
65. Vorinostat [mart.]
66. Vorinostat [who-dd]
67. Mls001065855
68. Mls006011941
69. Gtpl6852
70. Dtxsid6041133
71. Vorinostat [orange Book]
72. Bdbm19149
73. Vorinostat (saha; Mk0683)
74. Suberanilohydroxaminic Acid
75. 1t69
76. N-hydroxy-n''-phenyloctanediamide
77. Bcpp000018
78. Hms2219l20
79. Hms3264d20
80. Hms3327c12
81. Hms3426g03
82. Hms3650d09
83. Hms3654g11
84. Hms3715e22
85. Hms3745m03
86. Pharmakon1600-01502267
87. Bcp01858
88. Ex-a2745
89. Saha, >=98% (hplc)
90. Vorinostat,saha,zolinza,mk-0683
91. Zinc1543873
92. Tox21_112605
93. Tox21_113623
94. Vorinostat,cas:149647-78-9
95. Nsc748799
96. Nsc759852
97. Octanediamide, N1-hydroxy-n8-phenyl
98. S1047
99. Sk1390
100. Akos015966648
101. Octanediamide, N1-hydroxy-n8-phenyl-
102. Tox21_112605_1
103. Ac-1923
104. Ccg-208659
105. Cs-0589
106. Db02546
107. Dg-0025
108. Nsc 701852
109. Nsc 748799
110. Nsc 759852
111. Sb17319
112. Suberoylanilide Hydroxamic Acid (saha)
113. Ncgc00168085-03
114. Ncgc00168085-04
115. Ncgc00168085-05
116. Ncgc00168085-13
117. Bp-25652
118. Bp-30216
119. Bv164560
120. Hy-10221
121. Sy009383
122. Am20030018
123. Ft-0650593
124. H1388
125. Sw199536-4
126. Ec-000.2057
127. D06320
128. Ab00375377-07
129. Ab00375377-08
130. Ab00375377-09
131. Ab01644613_25
132. 647s789
133. A808935
134. Q905901
135. Vorinostat, Saha, Suberoylanilide Hydroxamic Acid
136. Sr-05000000373-2
137. Sr-05000000373-6
138. Sr-05000000373-8
139. W-201327
140. Brd-k81418486-001-01-2
141. Brd-k81418486-001-10-3
142. Brd-k81418486-001-12-9
143. Brd-k81418486-001-13-7
144. Brd-k81418486-001-17-8
145. Brd-k81418486-001-18-6
146. Z1541632802
147. N-hydroxy-n Inverted Exclamation Mark -phenyloctanediamide
148. 1227736-21-1
| Molecular Weight | 264.32 g/mol |
|---|---|
| Molecular Formula | C14H20N2O3 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 264.14739250 g/mol |
| Monoisotopic Mass | 264.14739250 g/mol |
| Topological Polar Surface Area | 78.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 276 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Zolinza |
| PubMed Health | Vorinostat (By mouth) |
| Drug Classes | Antineoplastic Agent |
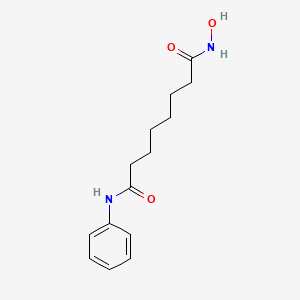
| Drug Label | ZOLINZA contains vorinostat, which is described chemically as N-hydroxy-N'-phenyloctanediamide. The empirical formula is C14H20N2O3. The molecular weight is 264.32 and the structural formula is:Vorinostat is a white to light orange powder. It is very |
| Active Ingredient | Vorinostat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Merck |
| 2 of 2 | |
|---|---|
| Drug Name | Zolinza |
| PubMed Health | Vorinostat (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | ZOLINZA contains vorinostat, which is described chemically as N-hydroxy-N'-phenyloctanediamide. The empirical formula is C14H20N2O3. The molecular weight is 264.32 and the structural formula is:Vorinostat is a white to light orange powder. It is very |
| Active Ingredient | Vorinostat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Merck |
Antineoplastic Agents; Histone Deacetylase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Vorinostat is indicated for the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma who have progressive, persistent or recurrent disease on or following two systemic therapies. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
Risk of pulmonary embolism and deep-vein thrombosis. Clinicians should be alert to signs and symptoms of such effects, especially in patients with a prior history of thromboembolic events.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1275
Risk of dose-related thrombocytopenia and anemia. Dosage should be adjusted or therapy discontinued if thrombocytopenia or anemia occurs.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1275
Risk of nausea, vomiting, and diarrhea; antiemetic and/or antidiarrheal agents may be required. To prevent dehydration, fluid and electrolyte replacement should be administered. Preexisting nausea, vomiting, and diarrhea should be adequately controlled before initiating therapy.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1275
Risk of hyperglycemia. Serum glucose concentrations should be monitored, especially in patients with known or possible diabetes mellitus. Diet and/or antidiabetic therapy should be adjusted, if needed.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1275
For more Drug Warnings (Complete) data for Vorinostat (23 total), please visit the HSDB record page.
For the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma who have progressive, persistent or recurrent disease on or following two systemic therapies.
Malignant pleural mesothelioma, Treatment of Cutaneous T-Cell Lymphoma
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Histone Deacetylase Inhibitors
Compounds that inhibit HISTONE DEACETYLASES. This class of drugs may influence gene expression by increasing the level of acetylated HISTONES in specific CHROMATIN domains. (See all compounds classified as Histone Deacetylase Inhibitors.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XH - Histone deacetylase (hdac) inhibitors
L01XH01 - Vorinostat
Route of Elimination
In vitro studies using human liver microsomes indicate negligible biotransformation by cytochromes P450 (CYP). Vorinostat is eliminated predominantly through metabolism with less than 1% of the dose recovered as unchanged drug in urine, indicating that renal excretion does not play a role in the elimination of vorinostat. However, renal excretion does not play a role in the elimination of vorinostat.
The pharmacokinetics of vorinostat were evaluated in 23 patients with relapsed or refractory advanced cancer. After oral administration of a single 400-mg dose of vorinostat with a high-fat meal, the mean +/- standard deviation area under the curve (AUC) and peak serum concentration (Cmax) and the median (range) time to maximum concentration (Tmax) were 5.5+/-1.8 uM.hr, 1.2+/-0.62 uM and 4 (2-10) hours, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
In the fasted state, oral administration of a single 400-mg dose of vorinostat resulted in a mean AUC and Cmax and median Tmax of 4.2+/-1.9 uM.hr and 1.2+/-0.35 uM and 1.5 (0.5-10) hours, respectively. Therefore, oral administration of vorinostat with a high-fat meal resulted in an increase (33%) in the extent of absorption and a modest decrease in the rate of absorption (Tmax delayed 2.5 hours) compared to the fasted state. However, these small effects are not expected to be clinically meaningful. In clinical trials of patients with CTCL, vorinostat was taken with food.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
At steady state in the fed-state, oral administration of multiple 400-mg doses of vorinostat resulted in a mean AUC and Cmax and a median Tmax of 6.0+/-2.0 uM.hr, 1.2+/-0.53 uM and 4 (0.5-14) hours, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
Vorinostat is approximately 71% bound to human plasma proteins over the range of concentrations of 0.5 to 50 ug/mL.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
For more Absorption, Distribution and Excretion (Complete) data for Vorinostat (9 total), please visit the HSDB record page.
The major pathways of vorinostat metabolism involve glucuronidation and hydrolysis followed by -oxidation. Human serum levels of two metabolites, O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were measured. Both metabolites are pharmacologically inactive. Compared to vorinostat, the mean steady state serum exposures in humans of the O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were 4-fold and 13-fold higher, respectively. In vitro studies using human liver microsomes indicate negligible biotransformation by cytochromes P450 (CYP).
Vorinostat is extensively metabolized to inactive metabolites, principally by glucuronidation and hydrolysis followed by beta-oxidation. The drug is not metabolized by cytochrome P-450 (CYP) isoenzymes.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1276
The major pathways of vorinostat metabolism involve glucuronidation and hydrolysis followed by beta-oxidation. Human serum levels of two metabolites, O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were measured. Both metabolites are pharmacologically inactive. Compared to vorinostat, the mean steady state serum exposures in humans of the O-glucuronide of vorinostat and 4-anilino-4-oxobutanoic acid were 4-fold and 13-fold higher, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
The mean urinary recovery of two pharmacologically inactive metabolites at steady state was 16+/-5.8% of vorinostat dose as the O glucuronide of vorinostat, and 36+/-8.6% of vorinostat dose as 4-anilino-4-oxobutanoic acid. Total urinary recovery of vorinostat and these two metabolites averaged 52+/-13.3% of vorinostat dose.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
2 hours
... Patients (n = 23) received single doses of 400 mg vorinostat on day 1 (fasted) and day 5 (fed) with 48 hours of pharmacokinetic sampling on both days. Patients received 400 mg vorinostat once daily on days 7 to 28. On day 28, vorinostat was given (fed) with pharmacokinetic sampling for 24 hours after dose. The apparent t(1/2) of vorinostat was short (approximately 1.5 hours). ...
PMID:17145826 Rubin EH et al; Clin Cancer Res 12 (23): 7039-45 (2006)
The mean terminal half-life was /approximately/ 2.0 hours for both vorinostat and the O-glucuronide metabolite, while that of the 4-anilino-4-oxobutanoic acid metabolite was 11 hours.
US Natl Inst Health; DailyMed. Current Medication Information for ZOLINZA (vorinostat) capsule (October 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cd86ee78-2781-468b-930c-3c4677bcc092
Vorinostat inhibits the enzymatic activity of histone deacetylases HDAC1, HDAC2 and HDAC3 (Class I) and HDAC6 (Class II) at nanomolar concentrations (IC50< 86 nM). These enzymes catalyze the removal of acetyl groups from the lysine residues of histones proteins. In some cancer cells, there is an overexpression of HDACs, or an aberrant recruitment of HDACs to oncogenic transcription factors causing hypoacetylation of core nucleosomal histones. By inhibiting histone deacetylase, vorinostat causes the accumulation of acetylated histones and induces cell cycle arrest and/or apoptosis of some transformed cells. The mechanism of the antineoplastic effect of vorinostat has not been fully characterized.
Vorinostat, a histone deacetylase inhibitor, is an antineoplastic agent. The mechanism of the antineoplastic effect of vorinostat has not been fully characterized. Vorinostat inhibits the enzymatic activity of histone deacetylases HDAC1, HDAC2, and HDAC3 (Class I) and HDAC6 (Class II) at nanomolar concentrations. HDAC enzymes catalyze the removal of acetyl groups from the lysine residues of proteins, including histones and transcription factors. Overexpression of HDAC enzymes or aberrant recruitment of HDAC enzymes to oncogenic transcription factors causing hypoacetylation of core nucleosomal histones has been observed in some cancer cells. Hypoacetylation of histones is associated with a condensed chromatin structure and repression of gene transcription. Inhibition of HDAC activity allows for the accumulation of acetyl groups on the histone lysine residues, resulting in an open chromatin structure and transcriptional activation. In vitro, vorinostat causes the accumulation of acetylated histones and induces cell cycle arrest and/or apoptosis of some transformed cells.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1276
Although the pathophysiological processes involved in dopamine (DA) neuron degeneration in Parkinson's disease (PD) are not completely known, apoptotic cell death has been suggested to be involved and can be modeled in DAergic cell lines using the mitochondrial toxin 1-methyl-4-phenylpyridinium (MPP(+)). Recently, it has been suggested that histone deacetylase inhibitors (HDACIs) may reduce apoptotic cell death in various model systems. However, their utility in interfering with DA cell death remains unclear. The HDACIs sodium butyrate (NaB), valproate (VPA) and suberoylanilide hydroxamic acid (SAHA) were tested for their ability to prevent MPP(+)-mediated cytotoxicity in human derived SK-N-SH and rat derived MES 23.5 cells. All three HDACIs at least partially prevented MPP(+)-mediated apoptotic cell death. The protective effects of these HDACIs coincided with significant increases in histone acetylation. These results suggest that HDACIs may be potentially neuroprotective against DA cell death ...
PMID:20654591 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2941155 Kidd SK, Schneider JS; Brain Res 1354: 172-8 (2010)
Histone deacetylase inhibitors (HDACi) developed as anti-cancer agents have a high degree of selectivity for killing cancer cells. HDACi induce acetylation of histones and nonhistone proteins, which affect gene expression, cell cycle progression, cell migration, and cell death. The mechanism of the tumor selective action of HDACi is unclear. Here, /the authors/ show that the HDACi, vorinostat (Suberoylanilide hydroxamic acid, SAHA), induces DNA double-strand breaks (DSBs) in normal (HFS) and cancer (LNCaP, A549) cells. Normal cells in contrast to cancer cells repair the DSBs despite continued culture with vorinostat. In transformed cells, phosphorylated H2AX (gammaH2AX), a marker of DNA DSBs, levels increased with continued culture with vorinostat, whereas in normal cells, this marker decreased with time. Vorinostat induced the accumulation of acetylated histones within 30 min, which could alter chromatin structure-exposing DNA to damage. After a 24-hr culture of cells with vorinostat, and reculture without the HDACi, gammaH2AX was undetectable by 2 hr in normal cells, while persisting in transformed cells for the duration of culture. Further, /investigators/ found that vorinostat suppressed DNA DSB repair proteins, e.g., RAD50, MRE11, in cancer but not normal cells. Thus, the HDACi, vorinostat, induces DNA damage which normal but not cancer cells can repair. This DNA damage is associated with cancer cell death. These findings can explain, in part, the selectivity of vorinostat in causing cancer cell death at concentrations that cause little or no normal cell death.
PMID:20679231 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2930422 Lee JH et al; Proc Natl Acad Sci U S A 107 (33): 14639-44 (2010)
... Some histone deacetylase inhibitors, such as trichostatin A and scriptaid, have improved the full-term development of mouse clones significantly, but the mechanisms allowing for this are unclear. Here, /the authors/ found that two other specific inhibitors, suberoylanilide hydroxamic acid and oxamflatin, could also reduce the rate of apoptosis in blastocysts, improve the full-term development of cloned mice, and increase establishment of nuclear transfer-generated embryonic stem cell lines significantly without leading to obvious abnormalities. However, another inhibitor, valproic acid, could not improve cloning efficiency. Suberoylanilide hydroxamic acid, oxamflatin, trichostatin A, and scriptaid are inhibitors for classes I and IIa/b histone deacetylase, whereas valproic acid is an inhibitor for classes I and IIa, suggesting that inhibiting class IIb histone deacetylase is an important step for reprogramming mouse cloning efficiency.
PMID:20686182 Ono T et al; Biol Reprod 83 (6): 929-37 (2010)
For more Mechanism of Action (Complete) data for Vorinostat (23 total), please visit the HSDB record page.