

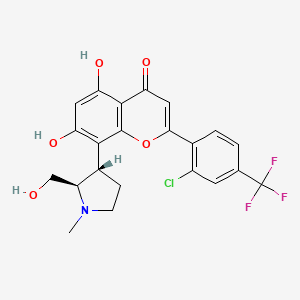

1. 2-(2-chloro-4-(trifluoromethyl) Phenyl)-5, 7-dihydroxy-8-((2r, 3s)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4h-1-benzopyran-4-one
1. 1000023-04-0
2. Voruciclib [inn]
3. P1446a-05
4. W66xp666am
5. 2-[2-chloro-4-(trifluoromethyl)phenyl]-5,7-dihydroxy-8-[(2r,3s)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl]chromen-4-one
6. Chembl3905910
7. 2-(2-chloro-4-(trifluoromethyl)phenyl)-5,7-dihydroxy-8-((2r,3s)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4h-1-benzopyran-4-one
8. 4h-1-benzopyran-4-one, 2-(2-chloro-4-(trifluoromethyl)phenyl)-5,7-dihydroxy-8-((2r,3s)-2-(hydroxymethyl)-1-methyl-3-pyrrolidinyl)-
9. Unii-w66xp666am
10. Voruciclib [who-dd]
11. Gtpl9923
12. Schembl3108205
13. Ex-a1056
14. Bdbm50193104
15. Nsc784284
16. P1446a
17. Db15157
18. Nsc-784284
19. Ncgc00387877-01
20. Ac-31553
21. As-82346
22. Hy-12422
23. Cs-0011273
24. Q27292377
25. 2-(2-chloro-4-(trifluoromethyl)phenyl)-5,7-dihydroxy-8-((2r,3s)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4h-chromen-4-one
26. 2-[2-chloro-4-(trifluoromethyl)phenyl]-5,7-dihydroxy-8-[(2r,3s)-2-(hydroxymethyl)-1-methyl-3-pyrrolidinyl]-4h-1-benzopyran-4-one
| Molecular Weight | 469.8 g/mol |
|---|---|
| Molecular Formula | C22H19ClF3NO5 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Exact Mass | 469.0903849 g/mol |
| Monoisotopic Mass | 469.0903849 g/mol |
| Topological Polar Surface Area | 90.2 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 750 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)