
1. Ademol
1. Ademol
2. Trifluoromethylthiazide
3. Flumethiazid
4. Flumetiazid
5. 148-56-1
6. Fludemil
7. Ademil
8. Routrax
9. Trifluomethylthiazide
10. Nsc 44626
11. Trifluoromethyl Thiazide
12. Nsc-44626
13. 3pa0cds0m5
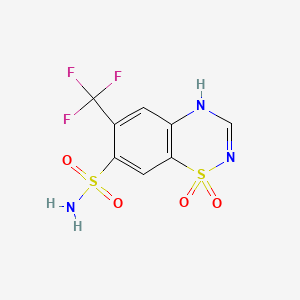
14. 6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
15. 4h-1,2,4-benzothiadiazine-7-sulfonamide, 6-(trifluoromethyl)-, 1,1-dioxide
16. Flumethiazide (inn)
17. Flumethiazide [inn]
18. Flumetiazide [dcit]
19. Flumethazide
20. Flumethiazidum
21. Flumetiazida
22. Flumetiazide
23. Flumethiazide [inn:ban]
24. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 6-(trifluoromethyl)-, 1,1-dioxide
25. Flumethiazidum [inn-latin]
26. Flumetiazida [inn-spanish]
27. Hsdb 2845
28. Einecs 205-717-4
29. Unii-3pa0cds0m5
30. Ademol (tn)
31. 6-trifluoromethyl-7-sulfamyl-1,2,4-benzothiadiazine 1,1-dioxide
32. 6-trifluoromethyl-7-sulfamyl-1,2,4-benzothiadiazine-1,1-dioxide
33. 6-(trifluoromethyl)-1,2,4-benzo-thiadiazine-7-sulfonamide 1,1-dioxide
34. 6-(trifluoromethyl)-1,4,2-benzothiadiazine-7-sulfonamido 1,1-dioxide
35. 6-trifluoromethyl-7-sulfamoyl-4h-1,2,4-benzothiadiazine 1,1-dioxide
36. 6-trifluoromethyl-7-sulfamoyl-4h-1,4,2-benzothiadiazine 1,1-dioxide
37. 7-sulfamoyl-6-trifluoromethyl-2h-1,2,4-benzothiadiazine 1,1-dioxide
38. Flumethiazide [mi]
39. Flumethiazide [hsdb]
40. Schembl26534
41. Flumethiazide [vandf]
42. Flumethiazide [who-dd]
43. Chembl2105128
44. Dtxsid60163862
45. Chebi:135404
46. Nsc44626
47. Wln: T66 Bswm Enj Hxfff Iszw
48. D02453
49. Q27257858
50. 6-trifluoromethyl-7-sulfamyl-1,4-benzothiadiazine 1,1-dioxide
51. 6-(trifluoromethyl)-1,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
52. 6-(trifluoromethyl)-1,4-benzothiadiazine-7-sulfonamido 1,1-dioxide
53. 6-trifluoromethyl-7-sulfamoyl-1,2,4-benzothiadiazine-1,1-dioxide
54. 6-trifluoromethyl-7-sulfamoyl-4h-1,4-benzothiadiazine 1,1-dioxide
55. 7-sulfamoyl-6-trifluoromethyl-2h-1,4-benzothiadiazine 1,1-dioxide
56. 1,1-dioxo-6-(trifluoromethyl)-4h-benzo[e][1,2,4]thiadiazine-7-sulfonamide
57. 2h-1,4-benzothiadiazine-7-sulfonamide, 6-(trifluoromethyl)-, 1,1-dioxide
58. 4h-1,4-benzothiadiazine-7-sulfonamide, 6-(trifluoromethyl)-, 1,1-dioxide
59. 1,1-dioxo-6-(trifluoromethyl)-4h-1lambda6,2,4-benzothiadiazine-7-sulfonamide
| Molecular Weight | 329.3 g/mol |
|---|---|
| Molecular Formula | C8H6F3N3O4S2 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 1 |
| Exact Mass | 328.97518251 g/mol |
| Monoisotopic Mass | 328.97518251 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 617 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...AVAILABLE AS TABLETS FOR ORAL ADMIN. ...GIVEN IN DIVIDED DAILY DOSES FOR TREATMENT OF HYPERTENSION, BUT SINGLE DAILY DOSE MAY BE PREFERABLE FOR MOBILIZATION OF EDEMA FLUID. ... LESS COMMON USAGES...INCL TREATMENT OF DIABETES INSIPIDUS & MGMNT OF HYPERCALCIURIA IN PT WITH...URINARY CALCULI... /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 832
THIAZIDES HAVE THEIR GREATEST USEFULNESS AS DIURETICS IN MGMNT OF EDEMA OF CHRONIC CARDIAC DECOMPENSATION. EDEMA DUE TO CHRONIC HEPATIC OR RENAL DISEASE ALSO RESPONDS FAVORABLY. WHEN EMPLOYED IN HYPERTENSIVE DISEASE, WITH OR WITHOUT OVERT EDEMA, THESE AGENTS HAVE HYPOTENSIVE ACTION... /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 832
PLASMA POTASSIUM CONCN SHOULD BE DETERMINED PERIODICALLY IN PT WHO RECEIVE THIAZIDE DIURETICS FOR EXTENDED PERIODS. ...TO AVOID NEGATIVE POTASSIUM BALANCE, THIAZIDES ARE OFTEN PRESCRIBED IN COMBINATION WITH POTASSIUM CHLORIDE. /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 832
WHEN CARDIAC DECOMPENSATION OR HYPERTENSION IS ACCOMPANIED BY SIGNIFICANT IMPAIRMENT OF RENAL FUNCTION, THIAZIDES SHOULD BE ADMIN WITH CAUTION BECAUSE OF THEIR CAPACITY TO AGGRAVATE RENAL INSUFFICIENCY.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 832
THIAZIDES ARE ABSORBED FROM GASTROINTESTINAL TRACT & OWE THEIR USEFULNESS LARGELY TO THEIR EFFECTIVENESS BY ORAL ROUTE. ABSORPTION IS RELATIVELY RAPID. ... DRUG PASSES READILY THROUGH PLACENTAL BARRIER TO FETUS. ... MOST COMPD ARE RAPIDLY EXCRETED WITHIN 3-6 HR. /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 831
INHIBITION OF CARBONIC ANHYDRASE DECR CONCN OF HYDROGEN ION AVAILABLE FOR EXCHANGE. DECR HYDROGEN-ION CONCN LEADS TO NATRIURESIS & THEREFORE DIURESIS. USE OF CARBONIC ANHYDRASE INHIBITOR DOES NOT GREATLY ALTER CHLORIDE EXCRETION. HOWEVER, THERE MAY BE MARKED POTASSIUM LOSS WITH RESULTING HYPOKALEMIA. /DIURETICS/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 418
DOMINANT ACTION OF THIAZIDES IS TO INCR RENAL EXCRETION OF SODIUM & CHLORIDE & ACCOMPANYING VOL OF WATER. UNLIKE BOTH MERCURIALS & CARBONIC ANHYDRASE INHIBITORS, ACTION OF THIAZIDES IS VIRTUALLY INDEPENDENT OF ACID-BASE BALANCE. THIAZIDES ALSO EVOKE SIGNIFICANT AUGMENTATION OF POTASSIUM EXCRETION... /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 830
THIAZIDES ARE...SECRETED INTO TUBULAR FLUID BY ACTIVE PROCESS LOCATED IN PROXIMAL SEGMENT. ... THIAZIDES INHIBIT REABSORPTION OF SODIUM & ITS ATTENDANT ANION, CHLORIDE, IN DISTAL SEGMENT. ... AUGMENTED EXCRETION OF.../POTASSIUM/ RESULTS FROM ITS INCR SECRETION BY DISTAL TUBULE. /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 830