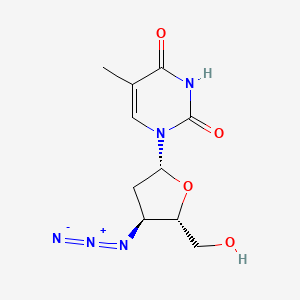

1. 3' Azido 2',3' Dideoxythymidine
2. 3' Azido 3' Deoxythymidine
3. 3'-azido-2',3'-dideoxythymidine
4. 3'-azido-3'-deoxythymidine
5. Antiviral Azt
6. Azidothymidine
7. Azt (antiviral)
8. Azt Antiviral
9. Azt, Antiviral
10. Bw A509u
11. Bwa 509u
12. Bwa-509u
13. Bwa509u
14. Retrovir
1. Azidothymidine
2. 30516-87-1
3. 3'-azido-3'-deoxythymidine
4. Retrovir
5. Azt
6. Zidovudinum
7. Thymidine, 3'-azido-3'-deoxy-
8. Compound S
9. Trizivir
10. Zidovudine [azt]
11. Zidovudina
12. Bw A509u
13. Zidovudin
14. Bwa509u
15. Zdv
16. 3'-deoxy-3'-azidothymidine
17. Bw-a509u
18. Bw-a-509u
19. Nsc 602670
20. 3'-azt
21. 1-((2r,4s,5s)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1h,3h)-dione
22. Chembl129
23. 1-(3-azido-2,3-dideoxy-beta-d-ribofuranosyl)thymine
24. 1-[(2r,4s,5s)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
25. Mls000028548
26. 4b9xt59t7s
27. Chebi:10110
28. Azidothymidine (azt)
29. Ncgc00023945-05
30. Zidovudinum [latin]
31. Smr000058351
32. Zidovudina [spanish]
33. Dsstox_cid_127
34. Dsstox_rid_75386
35. Dsstox_gsid_20127
36. 1-[(2r,4s,5s)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione
37. Propolis+azt
38. Nsc-602670
39. 3'-azido-2',3'-dideoxythymidine
40. Drg-0004
41. Retrovir(tm)
42. Azt & Li & Epo
43. Retrovir (tn)
44. Ccris 105
45. Cpd S
46. Intron A & Azt
47. Hsdb 6515
48. Racemic Liposomal Azt
49. Liposomal Azt-sn-1
50. Liposomal Azt-sn-3
51. Zidovudine+pro 140
52. Pc-sod+azt
53. Azt & Srcd4
54. Azt & Rifn.alpha.2
55. Azt & Rst4
56. Rifn-beta Seron & Azt
57. Mfcd00006536
58. 3'-azido-3'-deoxythymidine (aids)
59. Azt & Epo
60. Azt & Gm-csf
61. Azt & Hpa
62. Azt & Scd4
63. Azt & Sst
64. Zudovidine
65. Unii-4b9xt59t7s
66. Azt & Li & Gm-csf
67. Azt+pro 140
68. Met-sdf-1.beta. & Azt
69. Azt & Li & Il-1
70. Azt & Li & Il-6
71. Azt & Il-1
72. Azt & Il-2
73. Azt & Il-6
74. Azt & Interferon-.alpha.-2
75. Azt & Concanavalin A (cona)
76. Azt & Lymphoblastoid Interferon
77. Azt & Pm-19
78. Met-sdf-1.beta. & Zidovudine
79. 4lhm
80. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione (azt)
81. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione [azt]
82. Azt & Rscd4 & Rifn.alpha.a
83. 3'-azido-3'-deoxythymidine, Azt
84. Ds-4152 & Azt
85. 1-((2r,4r,5s)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1h,3h)-dione
86. 1-[(2r,4s,5s)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione
87. 5-methyl-1-[rac-(2r,4s,5s)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4-dione
88. Azt & Colony-stimulating Factor 2
89. Azt & Np (from Phca Or Hsa)
90. 3''-azido-thymidine
91. K7 [p Ti2 W10 O40]
92. Zidovudine [usan:usp:inn:ban:jan]
93. Zidovudine & Ifnl1
94. Zidovudine & Ifnl2
95. Zidovudine & Ifnl3
96. Zidovudine (retrovir)
97. Compound-s
98. Spectrum_001348
99. Azt & Cd4(178)-pe 40
100. Azt & Ifn.alpha.
101. Zidovudine & Il-29
102. Zidovudine [mi]
103. Zidovudine & Il-28a
104. Zidovudine & Il-28b
105. Zidovudine [inn]
106. Zidovudine [jan]
107. Azt & Interleukin 29
108. Opera_id_1602
109. Prestwick3_000333
110. Spectrum2_000927
111. Spectrum3_001507
112. Spectrum4_000332
113. Spectrum5_001101
114. 3'azido-3'deoxythymidine
115. Zidovudine [hsdb]
116. Zidovudine [iarc]
117. Zidovudine [usan]
118. 3'-azido-3'-deoxythymidine & Erythropoietin
119. 3'-azido-3'-deoxythymidine & Sho-saiko-to
120. Azidothymidine; Zidovudine
121. Interferon Ad + 3'-azido-3'-deoxythymidine
122. Azt & Interleukin 28a
123. Azt & Interleukin 28b
124. 3'-azido-3'-deoxythymidine & Concanavalin A
125. 3'-azido-3'-deoxythymidine & Interleukin-1
126. 3'-azido-3'-deoxythymidine & Interleukin-2
127. 3'-azido-3'-deoxythymidine & Interleukin-6
128. Zidovudine [vandf]
129. 3'-azido3'-deoxythymidine
130. Azt & Ifnl1
131. Azt & Ifnl2
132. Azt & Ifnl3
133. Zidovudine [mart.]
134. 1-(3-azido-2,3-dideoxy-beta-d-ribofuranosyl)-5-methylpyrimidine-2,4-(1h,3h)-dione
135. Azt & Interferon Lambda-1
136. Azt & Interferon Lambda-2
137. Azt & Interferon Lambda-3
138. Zidovudine [usp-rs]
139. Zidovudine [who-dd]
140. Zidovudine [who-ip]
141. 3''-deoxy-3-azidothymidine
142. Bspbio_000365
143. Bspbio_003153
144. Kbiogr_000703
145. Kbioss_001828
146. 399024-19-2
147. Mls001055351
148. Mls001076358
149. Mls002153202
150. Mls002222249
151. Zidovudine & Interleukin 29
152. Divk1c_000524
153. Spectrum1502109
154. Zidovudine [ema Epar]
155. 3'-deoxy-3'-azido-thymidine
156. Spbio_000834
157. Zidovudine & Interleukin 28a
158. Zidovudine & Interleukin 28b
159. Azt & Il-28a
160. Azt & Il-28b
161. Bpbio1_000403
162. Gtpl4825
163. Zidovudine (jp17/usp/inn)
164. 3'-azido-3'-deoxythymidine & Lithium & Erythropoietin
165. 3'-azido-3'-deoxythymidine & Lithium & Interleukin-1
166. 3'-azido-3'-deoxythymidine & Lithium & Interleukin-6
167. 3'-azido-3'-deoxythymidine & Lymphoblastoid Interferon
168. Dtxsid8020127
169. Schembl14615088
170. Sn-1-dipalmitoylglycerophospho-azt (in A Lipid Vesicle)
171. Sn-3-dipalmitoylglycerophospho-azt (in A Lipid Vesicle)
172. Zidovudine [ep Impurity]
173. Zidovudine [orange Book]
174. Azt & Il-29
175. Hms501k06
176. Kbio1_000524
177. Kbio2_001828
178. Kbio2_004396
179. Kbio2_006964
180. Kbio3_002653
181. 3'-azido-3'-deoxythymidine & Heteropolyoxotungstate Pm-19
182. Racemic-dipalmitoylglycerophospho-azt (in A Lipid Vesicle)
183. Zidovudine [ep Monograph]
184. 3''-azido-3''-deoxy-thymidine
185. Ninds_000524
186. Zidovudine & Interferon Lambda-1
187. Zidovudine & Interferon Lambda-2
188. Zidovudine & Interferon Lambda-3
189. Hms1921j20
190. Hms2090g11
191. Hms2092d06
192. Hms2096c07
193. Hms2234k17
194. Hms3259h17
195. Hms3713c07
196. Pharmakon1600-01502109
197. Zidovudine [usp Monograph]
198. Zidovudinum [who-ip Latin]
199. Combivir Component Zidovudine
200. Trizivir Component Zidovudine
201. Zinc3779042
202. 3''azido-2''3''-dideoxythymidine
203. Tox21_110062
204. Tox21_110894
205. Tox21_202203
206. Tox21_300578
207. Bbl033764
208. Bdbm50002692
209. Ccg-39924
210. Nsc758185
211. Stk801891
212. Akos005622576
213. Akos015842610
214. Tox21_110062_1
215. 3''-azido-2'',3''-dideoxythymidine
216. Db00495
217. Nc00666
218. Nsc-758185
219. Zidovudine Component Of Combivir
220. Zidovudine Component Of Trizivir
221. Idi1_000524
222. Ncgc00014918-01
223. Ncgc00023945-03
224. Ncgc00023945-04
225. Ncgc00023945-06
226. Ncgc00023945-07
227. Ncgc00023945-08
228. Ncgc00023945-09
229. Ncgc00023945-10
230. Ncgc00023945-12
231. Ncgc00023945-13
232. Ncgc00023945-24
233. Ncgc00023945-25
234. Ncgc00178237-01
235. Ncgc00178237-02
236. Ncgc00254276-01
237. Ncgc00259752-01
238. 1-((2r,4s,5s)-4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
239. As-13019
240. Sbi-0051731.p002
241. Propolis & Thymidine, 3'-azido-3'-deoxy-
242. S2579
243. Sw198799-2
244. En300-52518
245. 3'-azido-3'-deoxythymidine, >=98% (hplc)
246. C07210
247. D00413
248. D88500
249. 3'-azido-3'deoxythymidine & Interferon .alpha.
250. 3'-azido-3'-deoxythymidine, >=99.0% (hplc)
251. A820413
252. Q198504
253. Sr-01000000098
254. Sr-05000001587
255. J-700147
256. Sr-01000000098-3
257. Sr-05000001587-1
258. Brd-k72903603-001-04-6
259. Brd-k72903603-001-14-5
260. Lamivudine/zidovudine Teva Component Zidovudine
261. Z1723414428
262. Zidovudine Component Of Lamivudine/zidovudine Teva
263. Zidovudine, European Pharmacopoeia (ep) Reference Standard
264. 3'-azido-3'-deoxythymidine & Recombinant Interferon-.alpha.-2
265. Zidovudine, United States Pharmacopeia (usp) Reference Standard
266. 3'-azido-3'-deoxythymidine & Cd4-pseudomonas Exotoxin A Hybrid
267. Beta Interferon(rifn-beta Seron) & 3'-azido-3'-deoxythymidine(azt)
268. Lecithinized Superoxide Dismutase & Thymidine, 3'-azido-3'-deoxy-
269. 3'-azido-2',3'-dideoxythymidine & Scd4(soluble Recombinant Protein)
270. Sulfated Polysaccharide-peptidoglycan Ds-4152 & 3'-azido-3'-deoxythymidine
271. Thymidine, 3'-azido-3'-deoxy- & Pro 140 (anti-ccr5 Monoclonal Antibody)
272. Zidovudine, Pharmaceutical Secondary Standard; Certified Reference Material
273. (azt) 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
274. (azt)1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
275. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
276. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione(azt)
277. 3'-azido-3'-deoxythymidine & Granulocyte-macrophage Colony-stimulating Factor
278. 3'-azido-3'-deoxythymidine & Lithium & Granulocyte-macrophage Colony-stimulating Factor
279. 3'-azido-3'deoxythymidine & Recombinant Soluble Cd4 & Recombinant Interferon.alpha.a
280. 4-(4-azido-5-hydroxy-tetrahydro-furan-2-yl)-5-methyl-3h-pyrazine-2,6-dione
281. Met-stromal Cell-derived Factor-1.beta. (human) & 3'-azido-3'-deoxythymidine
282. 1-((2r,4s,5s)-4-(diazoamino)-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1h,3h)-dione
283. 1-((2r,4s,5s)-4-azido-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1h,3h)-dione
284. 1-((2r,5s)-4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
285. 1-((2s,4r,5r)-4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
286. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione (azddthd, Azt)
287. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione (n3ddthd)
288. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione(3''-azido-2'',3''-dideoxythymidine)
289. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione(azidothymidine, Azt)
290. 1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione(zidovudine, Azt)
291. 146426-54-2
292. 3'-azido-3'deoxythymidine & Nanoparticles (from Human Serum Albumin Or Polyhexylcyanoacrylate)
293. 3-((2s,3s,5r)-2-(hydroxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)tetrahydrofuran-3-yl)triaz-1-en-2-ium-1-ide
294. 3-azido-1,2,3-trideoxy-1-(3,4-dihydro-5-methyl-2,4-dioxo-1(2h)-pyrimidinyl)-d-erythro- Pentofuranuronic Acid
| Molecular Weight | 267.24 g/mol |
|---|---|
| Molecular Formula | C10H13N5O4 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 267.09675391 g/mol |
| Monoisotopic Mass | 267.09675391 g/mol |
| Topological Polar Surface Area | 93.2 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 484 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Retrovir |
| PubMed Health | Zidovudine |
| Drug Classes | Antiretroviral Agent |
| Drug Label | RETROVIR is the brand name for zidovudine (formerly called azidothymidine [AZT]), a pyrimidine nucleoside analogue active against HIV. RETROVIR IV Infusion is a sterile solution for intravenous infusion only. Each mL contains 10mg zidovudine in Wat... |
| Active Ingredient | Zidovudine |
| Dosage Form | Syrup; Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 50mg/5ml; 10mg/ml; 100mg |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 2 of 4 | |
|---|---|
| Drug Name | Zidovudine |
| PubMed Health | Zidovudine |
| Drug Classes | Antiretroviral Agent |
| Drug Label | RETROVIR is the brand name for zidovudine (formerly called azidothymidine [AZT]), a pyrimidine nucleoside analogue active against HIV. RETROVIR IV Infusion is a sterile solution for intravenous infusion only. Each mL contains 10mg zidovudine in Wat... |
| Active Ingredient | Zidovudine |
| Dosage Form | Tablet; Syrup; Capsule; Injectable |
| Route | oral; Injection; Oral |
| Strength | 50mg/5ml; 10mg/ml; 300mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Aurobindo; Hetero Labs Ltd Iii; Aurobindo Pharma; Cipla; Roxane; Sunshine Lake; Luitpold; Barr |
| 3 of 4 | |
|---|---|
| Drug Name | Retrovir |
| PubMed Health | Zidovudine |
| Drug Classes | Antiretroviral Agent |
| Drug Label | RETROVIR is the brand name for zidovudine (formerly called azidothymidine [AZT]), a pyrimidine nucleoside analogue active against HIV. RETROVIR IV Infusion is a sterile solution for intravenous infusion only. Each mL contains 10mg zidovudine in Wat... |
| Active Ingredient | Zidovudine |
| Dosage Form | Syrup; Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 50mg/5ml; 10mg/ml; 100mg |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 4 of 4 | |
|---|---|
| Drug Name | Zidovudine |
| PubMed Health | Zidovudine |
| Drug Classes | Antiretroviral Agent |
| Drug Label | RETROVIR is the brand name for zidovudine (formerly called azidothymidine [AZT]), a pyrimidine nucleoside analogue active against HIV. RETROVIR IV Infusion is a sterile solution for intravenous infusion only. Each mL contains 10mg zidovudine in Wat... |
| Active Ingredient | Zidovudine |
| Dosage Form | Tablet; Syrup; Capsule; Injectable |
| Route | oral; Injection; Oral |
| Strength | 50mg/5ml; 10mg/ml; 300mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Aurobindo; Hetero Labs Ltd Iii; Aurobindo Pharma; Cipla; Roxane; Sunshine Lake; Luitpold; Barr |
Anti-HIV Agents; Antimetabolites; Antimetabolites, Antineoplastic; Reverse Transcriptase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Zidovudine is indicated in combination with other antiretroviral agents for the treatment of HIV infection. ... /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2787
Zidovudine is indicated for the prevention of mother-to-child transmission of HIV-1 infection as part of a regimen that includes oral zidovudine beginning between 14 and 34 weeks gestation, continuous intravenous infusion of zidovudine during labor, and administration of zidovudine syrup to the neonate for the first 6 weeks of life. However, transmission to infants may still occur in some cases despite the use of this regimen. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2787
Zidovudine has been used prophylactically in health care workers at risk of acquiring HIV infection after occupational exposure to the virus. Risk of transmission from a single needlestick is approximately 0.3%. Efficacy, and optimal dose and duration of prophylactic treatment are unknown at this time; however, HIV infection has occurred in persons who received zidovudine prophylaxis after a needlestick or other parenteral exposure. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2787
For more Therapeutic Uses (Complete) data for ZIDOVUDINE (7 total), please visit the HSDB record page.
Adverse systemic effects reported with IV zidovudine are similar to those reported with oral zidovudine. However, clinical experience with IV zidovudine has been more limited than experience with oral zidovudine and the drug has generally been administered IV only for short periods of time. Long-term IV zidovudine therapy (i.e., longer than 2-4 weeks) has not been evaluated in adults and may enhance adverse hematologic effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 727
The most common adverse effects of zidovudine are hematologic effects (i.e., anemia, neutropenia), nausea, and headache. Because HIV-infected patients receiving zidovudine generally have serious underlying disease with multiple baseline symptomatology and clinical abnormalities and because many adverse effects that occurred in zidovudine-treated patients also occurred in patients receiving placebo, many reported effects may not be directly attributable to zidovudine. The frequency and severity of adverse effects associated with use of zidovudine in adults are greater in patients with more advanced disease at the time of initiation of therapy. In one study in asymptomatic patients receiving 100 mg of the drug orally 5 times daily for an average of longer than 1 year (range: 4 months to 2 years), only nausea occurred more frequently in patients receiving zidovudine than in those receiving placebo. Adverse effects reported with use of zidovudine in women, IV drug users, and racial minorities are similar to those reported with use of the drug in white males.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 727
Four patients with the acquired immunodeficiency syndrome, and a history of Pneumocystis carinii pneumonia developed severe pancytopenia (hemoglobin, less than 85 g/l; granulocytes, less than or equal to 0.5 X 10(9)/L; platelets, less than or equal to 30 X 10(9)/L) 12 to 17 weeks after the initiation of azidothymidine (AZT) therapy. The bone marrow was markedly hypocellular in three patients and moderately hypocellular in the fourth. Partial bone marrow recovery was documented within 4 to 5 weeks in three patients, but no marrow recovery has yet occurred in one patient during the more than 6 months since AZT treatment was discontinued.
PMID:3477107 Gill PS et al; Ann Intern Med 107 (4): 502-5 (1987)
Hematologic toxicity is causally related to zidovudine therapy, being directly related to dosage and duration of therapy with the drug, and has been reported most frequently in patients with advanced symptomatic HIV infection or low pretreatment hemoglobin concentrations, neutrophil counts, and helper/inducer (CD4+, T4+) T-cell counts. Patients with low serum folate or vitamin B12 concentrations may be at increased risk for developing bone marrow toxicity during zidovudine therapy. There also are limited data suggesting that bone marrow of patients with fulminant acquired immunodeficiency syndrome (AIDS) may be more sensitive to zidovudine-induced toxicity than that of patients with less advanced disease (e.g., AIDS-related complex (ARC)).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 727
For more Drug Warnings (Complete) data for ZIDOVUDINE (43 total), please visit the HSDB record page.
Used in combination with other antiretroviral agents for the treatment of human immunovirus (HIV) infections.
FDA Label
Trizivir is indicated for the treatment of human-immunodeficiency-virus (HIV) infection in adults.
This fixed combination replaces the three components (abacavir, lamivudine and zidovudine) used separately in similar dosages. It is recommended that treatment is started with abacavir, lamivudine,and zidovudine separately for the first six to eight weeks. The choice of this fixed combination should be based not only on potential adherence criteria, but mainly on expected efficacy and risk related to the three nucleoside analogues.
The demonstration of the benefit of Trizivir is mainly based on results of studies performed in treatment naive patients or moderately antiretroviral experienced patients with non-advanced disease.
In patients with high viral load (> 100,000 copies/ml) choice of therapy needs special consideration.
Overall, the virologic suppression with this triple nucleoside regimen could be inferior to that obtained with other multitherapies notably including boosted protease inhibitors or non-nucleoside reverse-transcriptase inhibitors, therefore the use of Trizivir should only be considered under special circumstances (e. g. co-infection with tuberculosis).
Before initiating treatment with abacavir, screening for carriage of the HLA-B*5701 allele should be performed in any HIV-infected patient, irrespective of racial origin. Screening is also recommended prior to re-initiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir (see 'management after an interruption of Trizivir therapy'). Abacavir should not be used in patients known to carry the HLA-B*5701 allele, unless no other therapeutic option is available in these patients, based on the treatment history and resistance testing.
Zidovudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Zidovudine is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. The lack of a 3'-OH group in the incorporated nucleoside analogue prevents the formation of the 5' to 3' phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AR04
J05AF01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF01 - Zidovudine
Absorption
Rapid and nearly complete absorption from the gastrointestinal tract following oral administration; however, because of first-pass metabolism, systemic bioavailability of zidovudine capsules and solution is approximately 65% (range, 52 to 75%). Bioavailability in neonates up to 14 days of age is approximately 89%, and it decreases to approximately 61% and 65% in neonates over 14 days of age and children 3 months to 12 years, respectively. Administration with a high-fat meal may decrease the rate and extent of absorption.
Route of Elimination
As in adult patients, the major route of elimination was by metabolism to GZDV. After intravenous dosing, about 29% of the dose was excreted in the urine unchanged and about 45% of the dose was excreted as GZDV.
Volume of Distribution
Apparent volume of distribution, HIV-infected patients, IV administration = 1.6 0.6 L/kg
Clearance
0.65+/- 0.29 L/hr/kg [HIV-infected, Birth to 14Days of Age]
1.14+/- 0.24 L/hr/kg [HIV-infected, 14Days to 3 Months of Age]
1.85 +/- 0.47 L/hr/kg [HIV-infected, 3 Months to 12Years of Age]. The transporters, ABCB1, ABCC4, ABCC5, and ABCG2 are involved with the clearance of zidovudine.
In patients with impaired renal function, plasma concentrations of zidovudine may be increased and the half-life prolonged. In one study in adults with impaired renal function (creatinine clearances ranging from 6-31 ml/minute) without HIV infections beta half life of zidovudine averaged 1.4 hours and was similar to that reported for adults with HIV infections who had normal renal function. However, the beta half life of glucuronide in these adults with impaired renal function averaged 8 hours and was considerably prolonged compared with that reported for adults with HIV infections who had normal renal function. In one study in adults with hemophilia and HIV infections who had elevated serum concentrations of aspartate aminotransferase (serum glutamic-oxaloacetic transaminase), alanine aminotransferase (serum glutamic-pyruvic transaminase), pharmacokinetics of zidovudine after a single 300 mg oral dose showed considerable interindividual variation.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 736
Following oral administration of zidovudine in patients with HIV infections, 63-95% of the dose is excreted in urine; approximately 14-18% of the dose is excreted as unchanged zidovudine and 72-74% is excreted as zidovudine 5'-O-glucuronide within 6 hours. Following iv administration of the drug in adults or children with HIV infections, approximately 18-29% of the dose is excreted in urine as unchanged drug and 45-60% is excreted as zidovudine 5'-O-glucuronide within 6 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 736
Zidovudine and 3'-azodp-3'-deoxy-5'-O-beta-d-glucopyranuronosylthymidine are eliminated principally in urine via both glomerular filtration and tubular secretion. Following oral or IV administration in adults with HIV infection, total body clearance of zidovudine averages 1.6 l/hr per kg (range: 0.8-2.7 l/hr per kg) and renal clearance of the drug averages 0.34 l/hr per kg. In children 3 months to 12 years of age, the total body clearance averaged 1.85 l/hr per kg. In one limited study in neonates and infants younger than 3 months of age, total body clearance of the drug averaged 0.65 l/hr per kg in those 14 days of age or younger and 1.14 l/hr per kg in those older than 14 days of age.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 736
Zidovudine is rapidly metabolized via glucuronidation in the liver to zidovudine 5'-O-glucuronide (GAZT); the metabolite has an apparent elimination half life of 1 hour (range: 0.6-1.7 hours). Zidovudine 5'-O-glucuronide does not appear to have antiviral activity against HIV.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 736
For more Absorption, Distribution and Excretion (Complete) data for ZIDOVUDINE (21 total), please visit the HSDB record page.
Hepatic. Metabolized by glucuronide conjugation to major, inactive metabolite, 3′-azido-3′-deoxy-5′- O-beta-D-glucopyranuronosylthymidine (GZDV). UGT2B7 is the primary UGT isoform that is responsible for glucuronidation. Compared to zidovudine, GZDV's area under the curve is approximately 3-fold greater. The cytochrome P450 isozymes are responsible for the reduction of the azido moiety to form 3'-amino-3'- deoxythymidine (AMT).
Zidovudine is rapidly metabolized via glucuronidation in the liver principally to 3-azido-3-deoxy-5-O-beta-d-glucopyranuronosylthymidine (GZDV; formerly GAZT); zidovudine is also metabolized to GZDV in renal microsomes. GZDV has an apparent elimination half-life of 1 hour (range: 0.6-1.7 hours) and does not appear to have antiviral activity against HIV. In addition, two other hepatic metabolites of zidovudine have been identified as 3-amino-3-deoxythymidine (AMT) and its glucuronide derivative (GAMT). Intracellularly, in both virus-infected and uninfected cells, zidovudine is converted to zidovudine monophosphate by cellular thymidine kinase; the monophosphate derivative is phosphorylated to zidovudine diphosphate via cellular dTMP kinase (thymidylate kinase) and then to zidovudine triphosphate via other cellular enzymes. Intracellular (host cell) conversion of zidovudine to the triphosphate derivative is necessary for the antiviral activity of the drug. Activation for antibacterial action, however, does not depend on phosphorylation within host cells but rather depends on conversion within bacterial cells.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 736
The mechanisms of intestinal mucosal transport and metabolism of zidovudine and other thymidine analogs were studied. No zidovudine metabolites appeared in any part of the gastrointestinal tract. Other thymidine analogs were rapidly metabolized in the upper gastrointestinal tract, but not in the colon.
PMID:1614965 Park GB, Mitra AK; Pharm Res 9 (Mar): 326-31 (1992)
Elimination half life, HIV-infected patients, IV administration = 1.1 hours (range of 0.5 - 2.9 hours)
The plasma half-life of zidovudine in adults averages approximately 0.53 hours following oral or IV administration. Following IV administration of zidovudine in adults or children, plasma concentrations of the drug appear to decline in a biphasic manner. Half-life in adults is less than 10 minutes in the initial phase and 1 hour in the terminal phase. Following IV administration over 1 hour of a single 80-, 120-, or 160-mg/sq m , dose in children 1-13 years of age with symptomatic HIV infection, the alpha half-life of zidovudine averaged 0.16-0.25 hours and the beta half-life averaged 1-1.7 hours. Plasma half-life of zidovudine generally is longer in neonates than in older children and adults but decreases with neonatal maturity. In one limited study in neonates and infants younger than 3 months of age, plasma half-life of zidovudine averaged 3.1 hours in those 14 days of age or younger and 1.9 hours in those older than 14 days of age. In a study in premature neonates (26-32 weeks gestation; birthweight 0.7-1.9 kg), the serum half-life of zidovudine averaged 7.3 hours at an average postnatal age of 6.3 days and averaged 4.4 hours at an average postnatal age of 17.7 days.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 736
The value for half-life of zidovudine is 1-2 hr.
PMID:2197818 Morse GD et al; DICP 24 (7-8): 754-60 (1990)
Zidovudine, a structural analog of thymidine, is a prodrug that must be phosphorylated to its active 5-triphosphate metabolite, zidovudine triphosphate (ZDV-TP). It inhibits the activity of HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation of the nucleotide analogue. It competes with the natural substrate dGTP and incorporates itself into viral DNA. It is also a weak inhibitor of cellular DNA polymerase and .
Zidovudine triphosphate can bind to and inhibit some mammalian cellular DNA polymerases, particularly beta- and gamma- polymerases, in vitro. However, zidovudine triphosphate appears to have a much greater affinity for viral RNA-directed DNA polymerase than for mammalian DNA polymerases. /Zidovudine triphosphate/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 734
... The antiretroviral activity of zidovudine appears to depend on intracellular conversion of the drug to a triphosphate metabolite; thus zidovudine triphosphate and not unchanged zidovudine appears to be the pharmacologically active form of the drug. Zidovudine is converted to zidovudine monophosphate by cellular thymidine kinase; the monophosphate is phosphorylated to zidovudine diphosphate via cellular thymidylate kinase and then to the triphosphate via other cellular enzymes. ... Conversion of the drug to the active triphosphate derivative occurs in both virus infected and uninfected cells. ... Zidovudine triphosphate appears to compete with thymidine triphosphate for viral RNA-directed DNA polymerase and incorporation into viral DNA. Following incorporation of zidovudine triphosphate into the viral DNA chain instead of thymidine triphosphate, DNA synthesis is prematurely terminated because the 3'-azido group of zidovudine prevents further 5' to 3' phosphodiester linkages. In addition, zidovudine monophosphate competitively inhibits thymidylate kinase, resulting in decreased formation of thymidine triphosphate; thus, the drug can decrease concentrations of this natural substrate for RNA-directed DNA polyerase and facilitate binding of zidovudine triphosphate to the enzyme. The drug also appears to decrease 2'-deoxycytidine triphosphate concentrations, but the mechanism of this effect is not known.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 733
... Antibacterial action of zidovudine appears to result from premature termination of bacterial DNA synthesis secondary to incorporation of phosphorylated zidovudine in the bacterial DNA chain. In vitro exposure of susceptible bacteria to the drug results in bacterial elongation and death secondary to cell lysis. ... The antibacterial action appears to depend on conversion of zidovudine to the active phosphorylated form via bacterial enzymes rather than via host enzymes. Zidovudine monophosphate, diphosphate, and triphosphate exhibit antibacterial activity in vitro with the triphosphate being most active and the monophosphate being least active. Susceptibility of bacteria to zidovudine appears to depend in large part on the presence of bacterial thymidine kinase. ... Organisms lacking thymidine kinase ... have been resistant to zidovudine, while those with relatively high concentrations of the enzyme ... have been highly susceptible to the drug; in addition, mutants resistant to the drug have had relatively low concentrations of the enzyme. The antibacterial activity of zidovudine also appears to depend in part on other factors such as permeability of the organism to the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 734
Zidovudine appears to alter nucleoside metabolism within host cells, resulting in decreased levels of thymidine triphosphate, 2'-deoxycytidine triphosphate, and several other deoxynucleoside triphosphates.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 734