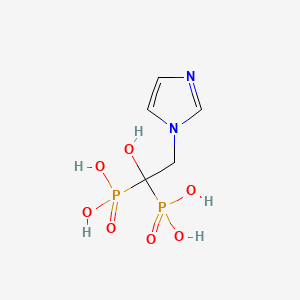

1. 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic Acid
2. Cgp 42'446
3. Cgp 42446
4. Cgp 42446a
5. Cgp-42'446
6. Cgp-42446
7. Cgp42'446
8. Cgp42446
9. Zoledronate
10. Zoledronic Acid Anhydrous
11. Zometa
1. Zoledronate
2. 118072-93-8
3. Zometa
4. Reclast
5. Aclasta
6. (1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyl)diphosphonic Acid
7. Cgp 42446
8. (1-hydroxy-2-imidazol-1-ylethylidene)diphosphonic Acid
9. Zoledronic Acid Anhydrous
10. Anhydrous Zoledronic Acid
11. (1-hydroxy-2-imidazol-1-yl-1-phosphonoethyl)phosphonic Acid
12. Phosphonic Acid, [1-hydroxy-2-(1h-imidazol-1-yl)ethylidene]bis-
13. Zol
14. [1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyl]bis(phosphonic Acid)
15. Orazol
16. Zol 446
17. Zoledronic Acid (inn)
18. Cgp-42446
19. Reclast (tn)
20. Zometa (tn)
21. Chembl924
22. Zoledronic Acid Teva
23. Zoledronic Acid, Anhydrous
24. Nsc-721517
25. Zoledronic Acid Medac
26. Chebi:46557
27. [1-hydroxy-2-(1h-imidazol-1-yl)-1-phosphonoethyl]phosphonic Acid
28. 70hz18ph24
29. Ncgc00159521-02
30. (1-hydroxy-2-(1h-imidazol-1-yl)ethylidene)bisphosphonic Acid
31. Cgp-42446a
32. Zoledronate Hydrate
33. Zoledronic Acid [usan:inn:ban]
34. Phosphonic Acid, (1-hydroxy-2-(1h-imidazol-1-yl)ethylidene)bis-
35. Zoladrona Acid Mylan
36. Zoledronic
37. Zoledronic Acid Accord
38. Zoledronic Acid [inn]
39. Zomera
40. 1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyldiphosphonic Acid
41. Bisphosphonate 3
42. Zometa (novartis)
43. Aclasta And Reclast
44. C5h10n2o7p2
45. [1-hydroxy-2-(1h-imidazol-1-yl)ethylidene]bisphosphonic Acid
46. Zoledronic-acid
47. Unii-70hz18ph24
48. Bph 91
49. [1-hydroxy-2-(1h-imidazol-1-yl)-ethylidene]bisphosphonic Acid
50. Dsstox_cid_22668
51. Dsstox_rid_80065
52. Zoledronic Acid, Zoledronate
53. Bidd:pxr0134
54. Dsstox_gsid_42668
55. Schembl19054
56. Zoledronic Acid [mi]
57. Bidd:gt0292
58. Zoledronic Acid (zoledronate)
59. Gtpl3177
60. Jmc515594 Compound 55
61. Dtxsid0042668
62. Bdbm12578
63. Cgp42446a
64. Zoledronic Acid [who-dd]
65. Hms2089o09
66. Bcp22750
67. Cgp-4244
68. Zinc3803652
69. Tox21_111739
70. Mfcd00867791
71. Nsc721517
72. S1314
73. Stl452893
74. Akos005145739
75. Ab07564
76. Ac-1092
77. Cs-1829
78. Db00399
79. Hs-0091
80. Nsc 721517
81. Ncgc00159521-03
82. Ncgc00159521-04
83. Ncgc00159521-05
84. Ncgc00159521-09
85. Ncgc00159521-18
86. Hy-13777
87. Cas-118072-93-8
88. Ft-0601384
89. Z0031
90. D08689
91. H11422
92. S00092
93. Ab01273947-01
94. Ab01273947-02
95. Ab01273947-03
96. Ab01273947_04
97. 072z938
98. A803876
99. Q218507
100. Sr-05000001436
101. Q-201946
102. Sr-05000001436-1
103. 1-hydroxy-2-(1-imidazolyl)ethane-1,1-diphosphonic Acid
104. Z1691545083
105. (1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyl)diphosphonicacid
| Molecular Weight | 272.09 g/mol |
|---|---|
| Molecular Formula | C5H10N2O7P2 |
| XLogP3 | -4.3 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 271.99632466 g/mol |
| Monoisotopic Mass | 271.99632466 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Reclast |
| Drug Label | Reclast contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formul... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml |
| Market Status | Prescription |
| Company | Novartis Pharms; Novartis |
| 2 of 6 | |
|---|---|
| Drug Name | Zoledronic acid |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zoledronic Acid Injection contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion); Injection; iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml; 4mmg; eq 4mg base/100ml; eq 4mg base/5ml; 4mg/100ml; 4mg/5ml(0.8mg/ml); eq 4mg base/vial |
| Market Status | Tentative Approval; Prescription |
| Company | Pharmaceutics; Hospira; Gland Pharma; Teva Parenteral; Apotex; Hikma Farmaceutica; Usv North America; Acs Dobfar Info Sa; Pharmaforce; Cipla; Sun Pharma Global; Emcure Pharms; Pharms; Dr Reddys Labs; Agila Speclts; Actavis; Akorn |
| 3 of 6 | |
|---|---|
| Drug Name | Zometa |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zometa contains zoledronicacid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronicacid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formu... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | Iv (infusion); iv (infusion) |
| Strength | eq 4mg base/100ml; eq 4mg base/5ml; eq 4mg base/vial |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 6 | |
|---|---|
| Drug Name | Reclast |
| Drug Label | Reclast contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formul... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml |
| Market Status | Prescription |
| Company | Novartis Pharms; Novartis |
| 5 of 6 | |
|---|---|
| Drug Name | Zoledronic acid |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zoledronic Acid Injection contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion); Injection; iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml; 4mmg; eq 4mg base/100ml; eq 4mg base/5ml; 4mg/100ml; 4mg/5ml(0.8mg/ml); eq 4mg base/vial |
| Market Status | Tentative Approval; Prescription |
| Company | Pharmaceutics; Hospira; Gland Pharma; Teva Parenteral; Apotex; Hikma Farmaceutica; Usv North America; Acs Dobfar Info Sa; Pharmaforce; Cipla; Sun Pharma Global; Emcure Pharms; Pharms; Dr Reddys Labs; Agila Speclts; Actavis; Akorn |
| 6 of 6 | |
|---|---|
| Drug Name | Zometa |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zometa contains zoledronicacid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronicacid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formu... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | Iv (infusion); iv (infusion) |
| Strength | eq 4mg base/100ml; eq 4mg base/5ml; eq 4mg base/vial |
| Market Status | Prescription |
| Company | Novartis |
Zoledronic acid is indicated to treat hypercalcemia of malignancy, multiple myeloma, bone metastases from solid tumors, osteoporosis in men and postmenopausal women, glucocorticoid induced osteoporosis, and Paget's disease of bone in men and women. Zoledronic acid is also indicated for the prevention of osteoporosis in post menopausal women and glucocorticoid induced osteoporosis.
Prevention of skeletal-related events and treatment of tumour-induced hypercalcaemia.
Prevention of skeletal-related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
Treatment of adult patients with tumour-induced hypercalcaemia.
* 4 mg / 5 ml and 4 mg / 100 ml: :
- Prevention of skeletal-related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
- Treatment of adult patients with tumour-induced hypercalcaemia (TIH).
* 5 mg / 100 ml: :
Treatment of osteoporosis:
- in post-menopausal women;
- in men;
at increased risk of fracture, including those with a recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy:
- in post-menopausal women;
- in men;
at increased risk of fracture.
Treatment of Paget's disease of the bone in adults.
Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
Treatment of adult patients with tumour-induced hypercalcaemia (TIH).
- Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone;
- treatment of adult patients with tumour-induced hypercalcaemia (TIH).
Treatment of osteoporosis:
- in post-menopausal women;
- in men;
at increased risk of fracture, including those with a recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy in post-menopausal women and in men at increased risk of fracture.
Treatment of Paget's disease of the bone.
Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
Treatment of adult patients with tumour-induced hypercalcaemia (TIH).
Treatment of osteoporosis:
- in post-menopausal women;
- in men;
at increased risk of fracture including those with a recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy:
- in post-menopausal women;
- in men;
at increased risk of fracture.
Treatment of Pagets disease of the bone in adults.
- Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in patients with advanced malignancies involving bone;
- treatment of tumour-induced hypercalcaemia (TIH);
- prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in patients with advanced malignancies involving bone;
- treatment of tumour-induced hypercalcaemia (TIH);
- prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone;
- treatment of adult patients with tumour-induced hypercalcaemia (TIH).
Treatment of osteoporosis
- in post-menopausal women
- in adult men
at increased risk of fracture, including those with recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy
- in post-menopausal women
- in adult men
at increased risk of fracture.
Treatment of Paget's disease of the bone in adults.
Osteogenesis imperfecta, Prevention of fracture and bone loss in postmenopausal women with early-stage breast cancer treated with aromatase inhibitors, Prevention of skeletal related events in patients with advanced malignancies involving bone, Tumour-induced hypercalcaemia
Treatment of osteoporosis, Treatment of Pagets disease of the bone
Zoledronic acid is a third generation, nitrogen containing bisphosphonate that inhibits osteoclast function and prevents bone resorption. The therapeutic window is wide as patients are unlikely to suffer severe effects from overdoses and the duration of action is long. Patients should be counselled regarding the risk of electrolyte deficiencies, renal impairment, osteonecrosis of the jaw, atypical femoral fractures, bronchoconstriction, hepatic impairment, hypocalcemia, and embryo-fetal toxicity.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M05 - Drugs for treatment of bone diseases
M05B - Drugs affecting bone structure and mineralization
M05BA - Bisphosphonates
M05BA08 - Zoledronic acid
Absorption
A 4mg intravenous dose reaches a Cmax of 37078.5ng/mL, with a Tmax of 0.3170.014h, and an AUC of 788181ng\*h/mL. A 5mg intravenous dose reaches a Cmax of 47176.1ng/mL, with a Tmax of 0.3680.005h, and an AUC of 917226ng\*h/mL.
Route of Elimination
Zoledronic acid is 39 16% eliminated in the urine as the unmetabolized parent drug.
Clearance
Zoledronic acid has a renal clearance of 3.7 2.0 L/h.
Zoledronic acid is not metabolized _in vivio_.
Zoledronic acid has a terminal elimination half life of 146 hours.
Bisphosphonates are taken into the bone where they bind to hydroxyapatite. Bone resorption by osteoclasts causes local acidification, releasing the bisphosphonate, which is taken into the osteoclast by fluid-phase endocytosis. Endocytic vesicles become acidified, releasing bisphosphonates into the cytosol of osteoclasts where they act. Osteoclasts mediate resorption of bone. When osteoclasts bind to bone they form podosomes, ring structures of F-actin. Etidronic acid also inhibits V-ATPases in the osteoclast, though the exact subunits are unknown, preventing F-actin from forming podosomes. Disruption of the podosomes causes osteoclasts to detach from bones, preventing bone resorption. Nitrogen containing bisphosphonates such as zoledronate are known to induce apoptosis of hematopoietic tumor cells by inhibiting the components of the mevalonate pathway farnesyl diphosphate synthase, farnesyl diphosphate, and geranylgeranyl diphosphate. These components are essential for post-translational prenylation of GTP-binding proteins like Rap1. The lack of prenylation of these proteins interferes with their function, and in the case of Rap1, leads to apoptosis. zoledronate also activated caspases which further contribute to apoptosis.