

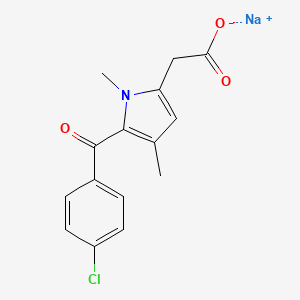

1. 5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrole-2-acetate Dihydrate
2. Mcn 2783-21-98
3. Zomax
4. Zomepirac
5. Zomepirac Potassium
1. Zomepirac Sodium Salt
2. 64092-48-4
3. Sodium Zomepirac
4. Zomepirac (sodium Salt)
5. Zomepirac Sodium Anhydrous
6. Sodium;2-[5-(4-chlorobenzoyl)-1,4-dimethylpyrrol-2-yl]acetate
7. Da5b6iwf46
8. Mcn-2783-21-98
9. 1h-pyrrole-2-acetic Acid, 5-(4-chlorobenzoyl)-1,4-dimethyl-, Sodium Salt
10. Ncgc00090751-01
11. Cas-64092-48-4
12. Dsstox_cid_13989
13. Dsstox_rid_79106
14. Dsstox_gsid_33989
15. Smr000686071
16. Mcn 2783-21-98
17. Ncgc00094811-01
18. Zomepiracsodiumsalt
19. Einecs 264-669-2
20. Unii-da5b6iwf46
21. Sodium 5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrole-2-acetate
22. 5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrole-2-acetic Acid Sodium Salt
23. Prestwick_973
24. Mls001055441
25. Mls002153996
26. Chembl266459
27. Dtxsid3033989
28. Schembl11172041
29. Zomepirac Sodium [who-dd]
30. Hms1570k20
31. Hms2097k20
32. Hms3714k20
33. Hy-b0890
34. Tox21_111006
35. Tox21_111335
36. Tox21_202177
37. Akos015962410
38. Tox21_111006_1
39. Ccg-220779
40. Cs-4353
41. Ncgc00017126-01
42. Ncgc00094811-06
43. Ncgc00259726-01
44. Ac-17496
45. Ft-0675929
46. Q27276294
47. Sodium [5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrol-2-yl]acetate
48. Sodium 2-(5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrol-2-yl)acetate
49. 1h-pyrrole-2-acetic Acid, 5-(4-chlorobenzoyl)-1,4-dimethyl-, Sodium Salt (1:1)
1. Zomepirac
| Molecular Weight | 313.71 g/mol |
|---|---|
| Molecular Formula | C15H13ClNNaO3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 313.0481653 g/mol |
| Monoisotopic Mass | 313.0481653 g/mol |
| Topological Polar Surface Area | 62.1 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 385 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)