

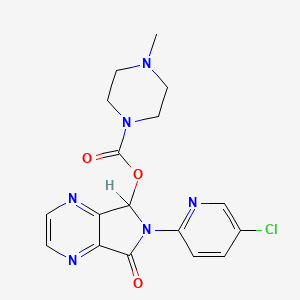

1. 6-(5-chloro-2-pyridyl)-6,7-dihydro- 7-oxo-5h-pyrrolo(3,4-b)pyrazin-5-yl 4-methyl-1- Piperazinecarboxylate
2. Imovane
3. Limovan
4. Nu-zopiclone
5. Optidorm
6. Ratio-zopiclone
7. Rhovane
8. Rp 27 267
9. Siaten
10. Somnosan
11. Ximovan
12. Zileze
13. Zimoclone
14. Zimovane
15. Zop
16. Zopi-puren
17. Zopicalm
18. Zopicalma
19. Zopiclodura
20. Zopiclon Abz
21. Zopiclon Al
22. Zopiclon Azu
23. Zopiclon Beta
24. Zopiclon Stada
25. Zopiclon Tad
26. Zopiclon Von Ct
27. Zopiclon-neuraxpharm
28. Zopiclon-ratiopharm
29. Zopiclon-teva
30. Zopitan
31. Zorclone
1. 43200-80-2
2. Imovane
3. Zimovane
4. Amoban
5. (+-)-zopiclone
6. Zopiclona
7. Zopiclonum
8. Zopiclonum [inn-latin]
9. Zopiclona [inn-spanish]
10. Rp-27267
11. [6-(5-chloropyridin-2-yl)-5-oxo-7h-pyrrolo[3,4-b]pyrazin-7-yl] 4-methylpiperazine-1-carboxylate
12. Rp 27267
13. 6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5h-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate
14. Chebi:32315
15. Amovane
16. Nsc-758463
17. Mls000083579
18. 03a5orl08q
19. 1-piperazinecarboxylic Acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5h-pyrrolo(3,4-b)pyrazin-5-yl Ester
20. 1-piperazinecarboxylic Acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5h-pyrrolo[3,4-b]pyrazin-5-yl Ester
21. 6-(5-chloro-2-pyridinyl)-7-oxo-6,7-dihydro-5h-pyrrolo[3,4-b]pyrazin-5-yl 4-methyl-1-piperazinecarboxylate
22. Ximovan
23. 4-methyl-1-piperazinecarboxylic Acid Ester With 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-hydroxy-5h-pyrrolo(3,4-b)pyrazin-5-one
24. Zopiclone (1.0 Mg/ml In Acetonitrile)
25. Smr000048685
26. Zopiclone [ban:inn:jan]
27. 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)carbonyloxy-7-oxo-6,7-dihydro-5h-pyrrolo[3,4-b]pyrazine
28. Zopiclone (tn)
29. Amoban (tn)
30. Zopiclone (jan/inn)
31. Sr-01000000090
32. Einecs 256-138-9
33. Dea No. 2784
34. Brn 0768704
35. Unii-03a5orl08q
36. Zopiclone [inn:ban:jan]
37. S-zopiclone
38. Zimovane Ls
39. 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)carbonyloxy-7-oxo-6,7-dihydro-5h-pyrrolo(3,4-b)pyrazine
40. Mfcd00133931
41. (+/-)-zopiclone
42. Zileze 7.5
43. Zileze 3.75
44. Zopiclone [inn]
45. Zopiclone [jan]
46. Zopiclone [mi]
47. Opera_id_1759
48. 27267 R.p.
49. Biomol-nt_000284
50. Zopiclone [mart.]
51. Z 4900
52. Zopiclone [who-dd]
53. Lopac0_001270
54. Schembl44419
55. Mls000028547
56. Mls001201837
57. Mls001304058
58. Bpbio1_001146
59. Chembl135400
60. Gtpl7430
61. Zopiclone [ep Impurity]
62. Dtxsid4041155
63. Zopiclone [ep Monograph]
64. Hms3267i08
65. Hms3713h22
66. Hms3746i05
67. Pharmakon1600-01503425
68. Bcp28513
69. Hy-b0741
70. Bbl010797
71. Bdbm50248251
72. Nsc758463
73. Stk599439
74. Zopiclone 1.0 Mg/ml In Acetonitrile
75. Akos005520380
76. Bcp9000663
77. Ccg-205343
78. Db01198
79. Nsc 758463
80. Ncgc00016108-04
81. Ncgc00016108-05
82. Ncgc00016108-06
83. Ncgc00024993-03
84. Ncgc00024993-04
85. Vs-02685
86. Sbi-0051236.p002
87. Eu-0101270
88. D01372
89. 200z802
90. Q220426
91. Sr-01000000090-2
92. Sr-01000000090-4
93. Sr-01000000090-6
94. Sr-01000000090-7
95. W-106239
96. Brd-a34309505-001-08-5
97. Brd-a34309505-001-10-1
98. Zopiclone, British Pharmacopoeia (bp) Reference Standard
99. Zopiclone, European Pharmacopoeia (ep) Reference Standard
100. (rs)-6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5h-pyrrolo[3,4-b]pyrazine-5-yl 4-methylpiperazine-1-carboxylate
101. 1169825-88-0
102. 4-methyl-1-piperazinecarboxylic Acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5h-pyrrolo(3,4-b)pyrazin-5-yl Ester
103. 4-methyl-1-piperazinecarboxylic Acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5h-pyrrolo[3,4-b] Pyrazin-5-yl Ester
104. 4-methyl-piperazine-1-carboxylic Acid 6-(5-chloro-pyridin-2-yl)-7-oxo-6,7-dihydro-5h-pyrrolo[3,4-b]pyrazin-5-yl Ester
105. 6-(5-chloropyridin-2-yl)-7-oxo-5h,6h,7h-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate
| Molecular Weight | 388.8 g/mol |
|---|---|
| Molecular Formula | C17H17ClN6O3 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 388.1050661 g/mol |
| Monoisotopic Mass | 388.1050661 g/mol |
| Topological Polar Surface Area | 91.8 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 573 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the short-term treatment of insomnia.
Zopiclone is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class and is indicated for the short-term treatment of insomnia. While Zopiclone is a hypnotic agent with a chemical structure unrelated to benzodiazepines, barbiturates, or other drugs with known hypnotic properties, it interacts with the gamma-aminobutyric acid-benzodiazepine (GABABZ) receptor complex. Subunit modulation of the GABABZ receptor chloride channel macromolecular complex is hypothesized to be responsible for some of the pharmacological properties of benzodiazepines, which include sedative, anxiolytic, muscle relaxant, and anticonvulsive effects in animal models. Zopiclone binds selectively to the brain alpha subunit of the GABA A omega-1 receptor.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N05CF01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CF - Benzodiazepine related drugs
N05CF01 - Zopiclone
Absorption
Rapidly absorbed following oral administration.
Extensively metabolized in the liver via decarboxylation (major pathway), demethylation, and side chain oxidation. Metabolites include an N-oxide derivative (weakly active; approximately 12% of a dose) and an N-desmethyl metabolite (inactive; approximately 16%). Approximately 50% of a dose is converted to other inactive metabolites via decarboxylation. Hepatic microsomal enzymes are apparently not involved in zopiclone clearance.
Elimination half life is approximately 5 hours (range 3.8 to 6.5 hours) and is prolonged to 11.9 hours in patients with hepatic insufficiency.
Zopiclone exerts its action by binding on the benzodiazepine receptor complex and modulation of the GABABZ receptor chloride channel macromolecular complex. Both zopiclone and benzodiazepines act indiscriminately at the benzodiazepine binding site on 1, 2, 3 and 5 GABAA containing receptors as full agonists causing an enhancement of the inhibitory actions of GABA to produce the therapeutic (hypnotic and anxiolytic) and adverse effects of zopiclone.