

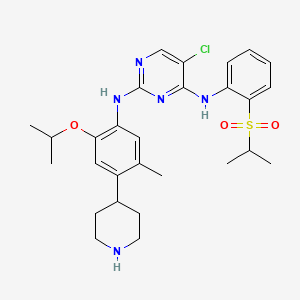

1. 5-chloro-n2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-n4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine
2. Ldk378
3. Zykadia
1. Ldk378
2. 1032900-25-6
3. Zykadia
4. Ldk-378
5. Nvp-ldk378-nx
6. Ldk 378
7. 5-chloro-n2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-n4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine
8. Ceritinib (ldk378)
9. Ceritinib(ldk378)
10. K418kg2get
11. Nvp-ldk-378-nx
12. Chembl2403108
13. Chebi:78432
14. 5-chloro-2-n-(5-methyl-4-piperidin-4-yl-2-propan-2-yloxyphenyl)-4-n-(2-propan-2-ylsulfonylphenyl)pyrimidine-2,4-diamine
15. 2,4-pyrimidinediamine, 5-chloro-n4-(2-((1-methylethyl)sulfonyl)phenyl)-n2-(5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl)-
16. 5-chloro-2-n-[5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl]-4-n-[2-(propane-2-sulfonyl)phenyl]pyrimidine-2,4-diamine
17. 5-chloro-n~2~-[5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl]-n~4~-[2-(propan-2-ylsulfonyl)phenyl]pyrimidine-2,4-diamine
18. 5-chloro-n2-(5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl)-n4-(2-(propane-2-sulfonyl)phenyl)pyrimidine-2,4-diamine
19. 5-chloro-n2-[2-isopropoxy-5-methyl-4-(4-piperidyl)phenyl]-n4-(2-isopropylsulfonylphenyl)pyrimidine-2,4-diamine
20. 5-chloro-n4-[2-[(1-methylethyl)sulfonyl]phenyl]-n2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine
21. 5-chloro-n4-[2-[(1methylethyl)sulfonyl]phenyl]-n2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine;5-chloro-n4-[2-[(1methylethyl)sulfonyl]phenyl]-n2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine
22. Ceritinib [usan:inn]
23. Unii-k418kg2get
24. Ceritinibum
25. Ceritinib[mi]
26. 5-chloro-n2-[5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl]-n4-[2-(propane-2-sulfonyl)phenyl]pyrimidine-2,4-diamine
27. Zykadia (tn)
28. Ldk378 Certinib
29. Ceritinib; Ldk378
30. Ldk378(ceritinib)
31. Ceritinib [inn]
32. Ceritinib [jan]
33. Eritinib (ldk378)
34. Ceritinib [mi]
35. Ceritinib [usan]
36. Ceritinib [vandf]
37. Ceritinib [who-dd]
38. Ceritinib (jan/usan/inn)
39. Gtpl7397
40. Schembl1014329
41. Ceritinib [orange Book]
42. Dtxsid10725373
43. Ex-a187
44. Hms3652h22
45. Hms3673i17
46. Hms3747a11
47. Amy10314
48. Bcp07611
49. Bdbm50436850
50. Mfcd26142648
51. Nsc776422
52. Nsc777193
53. Nsc800072
54. Zinc96272772
55. Akos025396438
56. Ccg-264762
57. Cs-1406
58. Db09063
59. Gs-6356
60. Nsc-776422
61. Nsc-777193
62. Nsc-800072
63. Sb16490
64. Compound 15b [pmid 23742252]
65. Ncgc00351603-10
66. Ncgc00351603-13
67. Ncgc00351603-15
68. Ac-27469
69. Hy-15656
70. Ft-0697208
71. S7083
72. Sw219725-1
73. D10551
74. A852144
75. J-690011
76. Q21011233
77. N-{2-methyl-5-[(methylamino)methyl]phenyl}-4-[(4-phenyl-2-quinazolinyl)amino]benzamide
78. 4mk
79. 5-chloro-n(2)-{5-methyl-4-(piperidin-4-yl)-2-[(propan-2-yl)oxy]phenyl}-n(4)-[2-(propane-2-sulfonyl)phenyl]pyrimidine-2,4-diamine
80. 5-chloro-n2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-n4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine
81. 5-chloro-n2-(5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl)-n4-(2-(propane-2- Sulfonyl)phenyl)pyrimidine-2,4-diamine
82. 5-chloro-n4-(2-((1-methylethyl)sulfonyl)phenyl)-n2-(5-methyl-2-(1-methylethoxy)-4- (piperidin-4-yl)phenyl)pyrimidine-2,4-diamine
83. Ceritinib;5-chloro-n2-[2-isopropoxy-5-methyl-4-(4-piperidyl)phenyl]-n4-(2-isopropylsulfonylphenyl)pyrimidine-2,4-diamine;ceritinib
84. N-{2-[(5-chloro-2-{[2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl]amino}pyrimidin-4-yl)amino]phenyl}propane-2-sulfonamide
| Molecular Weight | 558.1 g/mol |
|---|---|
| Molecular Formula | C28H36ClN5O3S |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 557.2227389 g/mol |
| Monoisotopic Mass | 557.2227389 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 835 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ceritinib is a kinase inhibitor indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. This indication is approved under accelerated approval based on tumor response rate and duration of response. An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
FDA Label
Zykadia is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK) positive advanced non small cell lung cancer (NSCLC) previously treated with crizotinib.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE
L01XE28
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01ED - Anaplastic lymphoma kinase (alk) inhibitors
L01ED02 - Ceritinib
Absorption
After oral administration of ceritinib, peak concentrations were achieved after approximately 4 to 6 hours.
Route of Elimination
Following oral administration of a single 750 mg radiolabeled ceritinib dose, 92.3% of the administered dose was recovered in the feces (with 68% as unchanged parent compound) while 1.3% of the administered dose was recovered in the urine.
Volume of Distribution
The apparent volume of distribution (Vd/F) is 4230 L following a single 750 mg dose.
Clearance
The geometric mean apparent clearance (CL/F) of ceritinib was lower at steady-state (33.2 L/h) after 750 mg daily dosing than after a single 750 mg dose (88.5 L/h).
In vitro studies demonstrated that CYP3A was the major enzyme involved in the metabolic clearance of ceritinib. Following oral administration of a single 750 mg radiolabeled ceritinib dose, ceritinib as the parent compound was the main circulating component (82%) in human plasma.
The terminal half life is 41 hours.
Ceritinib inhibits Anaplastic lymphoma kinase (ALK) also known as ALK tyrosine kinase receptor or CD246 (cluster of differentiation 246), which is an enzyme that in humans is encoded by the ALK gene. About 4-5% of NSCLCs have a chromosomal rearrangement that generates a fusion gene between EML4 (echinoderm microtubule-associated protein-like 4) and ALK (anaplastic lymphoma kinase), which results in constitutive kinase activity that contributes to carcinogenesis and seems to drive the malignant phenotype. Ceritinib exerts its therapeutic effect by inhibiting autophosphorylation of ALK, ALK-mediated phosphorylation of the downstream signaling protein STAT3, and proliferation of ALK-dependent cancer cells. Ceritinib has been shown to inhibit in vitro proliferation of cell lines expressing EML4-ALK and NPM-ALK fusion proteins and demonstrated dose-dependent inhibition of EML4-ALK-positive NSCLC xenograft growth in mice and rats.