
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
FDF
0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
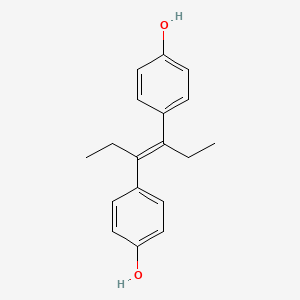

1. Agostilben
2. Apstil
3. Diethylstilbestrol, (z)-isomer
4. Diethylstilbestrol, Disodium Salt
5. Distilbne
6. Estrogen, Stilbene
7. Stilbene Estrogen
8. Stilbestrol
9. Tampovagan
1. 56-53-1
2. Stilbestrol
3. Stilboestrol
4. Distilbene
5. Agostilben
6. Estrobene
7. Estromenin
8. Stilbetin
9. Stilboestroform
10. Antigestil
11. Palestrol
12. Synestrin
13. Vagestrol
14. Fonatol
15. Diethylstilbesterol
16. Menostilbeen
17. Oestrogenine
18. Oestromensyl
19. Oestromienin
20. Stilbestrone
21. Synthoestrin
22. Comestrol
23. Cyren A
24. Domestrol
25. Dyestrol
26. Estrosyn
27. Grafestrol
28. Iscovesco
29. Microest
30. Milestrol
31. Oestromenin
32. Pabestrol
33. Sexocretin
34. Stilboefral
35. Stilbofolin
36. Synthofolin
37. Syntofolin
38. Diastyl
39. Makarol
40. Micrest
41. Serral
42. Stilkap
43. Bufon
44. Cyren
45. Desma
46. Sibol
47. Oekolp
48. Dawe's Destrol
49. Hi-bestrol
50. Di-estryl
51. Dietilestilbestrol
52. Neo-oestranol I
53. Rumestrol 1
54. Rumestrol 2
55. Stil-rol
56. Acnestrol
57. Climaterine
58. Dibestrol
59. Dicorvin
60. Gynopharm
61. Idroestril
62. Oestromensil
63. Oestromon
64. Protectona
65. Sedestran
66. Sintestrol
67. Stibilium
68. Tylosterone
69. Comestrol Estrobene
70. Stilbol
71. Diethylstilboesterol
72. Oestrol Vetag
73. Trans-diethylstilbestrol
74. Dibestrol 2 Premix
75. Bio-des
76. Estilbin Mco
77. (e)-diethylstilbestrol
78. Stil
79. Neo-oestranol 1
80. Tampovagan Stilboestrol
81. Des
82. Dibestrol '2' Premix
83. Trans-diethylstilbesterol
84. Synthestrin
85. (e)-3,4-bis(4-hydroxyphenyl)-3-hexene
86. Trans-diethylstilboesterol
87. Des (synthetic Estrogen)
88. Stilbestrol, Diethyl-
89. New-estranol 1
90. Diethylstilbestrolum
91. Percutatrine Oestrogenique Iscovesco
92. (e)-4,4'-(hex-3-ene-3,4-diyl)diphenol
93. 4,4'-dihydroxydiethylstilbene
94. Rcra Waste Number U089
95. 4-[(e)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol
96. Nsc-3070
97. 6898-97-1
98. Diethylstilbestrol (des)
99. Phenol, 4,4'-[(1e)-1,2-diethyl-1,2-ethenediyl]bis-
100. 4,4'-(3e)-hex-3-ene-3,4-diyldiphenol
101. Stilbestro
102. Cis-des
103. (e)-4,4'-(1,2-diethyl-1,2-ethenediyl)bisphenol
104. Phenol, 4,4'-(1,2-diethyl-1,2-ethenediyl)bis-, (e)-
105. Trans-4,4'-(1,2-diethyl-1,2-ethenediyl)bisphenol
106. 4,4'-dihydroxy-alpha,beta-diethylstilbene
107. Alpha,alpha'-diethyl-(e)-4,4'-stilbenediol
108. Chembl411
109. 4,4'-(hex-3-ene-3,4-diyl)diphenol
110. 731dca35bt
111. Phenol 4,4'-(1,2-diethyl-1,2-ethenediyl)bis-, (e)-
112. Tampovagan
113. Destrol
114. Chebi:41922
115. Estril
116. .alpha.,.alpha.'-diethylstilbenediol
117. Diethyl Stilbestrol
118. E-diethylstilbestrol
119. Strobene
120. Diaethylstilboestrolum
121. Estrogenine
122. Stilbestroform
123. Cis-diethylstilbesterol
124. Diethylstilbestrol, Mixture Of Cis And Trans
125. Dsstox_cid_465
126. 3,4-bis(p-hydroxyphenyl)-3-hexene
127. Dietilstilbestrolo
128. Dsstox_rid_75608
129. Dsstox_gsid_20465
130. .alpha.,.alpha.'-diethyl-(e)-4,4'-stilbenediol
131. Dietilstilbestrolo [dcit]
132. Dietilestilbestrol [spanish]
133. 4,4'-stilbenediol, .alpha.,.alpha.'-diethyl-, (e)-
134. Phenol, 4,4'-((1e)-1,2-diethyl-1,2-ethenediyl)bis-
135. Alpha,alpha'-diethylstilbenediol
136. Diethylstilbestrolum [inn-latin]
137. Dietilestilbestrol [inn-spanish]
138. Stilbestrol (tn)
139. Mg 137
140. Smr000058263
141. Ccris 240
142. Estilbin ''mco''
143. Hsdb 3060
144. Sr-01000745070
145. Einecs 200-278-5
146. Rcra Waste No. U089
147. Brn 2056095
148. Unii-731dca35bt
149. Bertrol
150. Diethylstilbestrol (usp/inn)
151. Trans-diethystilbesterol
152. Cis-alpha,alpha'-diethyl-4,4'-stilbenediol
153. 4,4'-(3-hexene-3,4-diyl)diphenol
154. 3erd
155. Cas-56-53-1
156. Ncgc00090749-04
157. Diethylstilbestrol [usp:inn:ban]
158. 4,4'-stilbenediol, Alpha,alpha'-diethyl-, (e)-
159. Prestwick_1070
160. (z)-4,4'-(1,2-diethyl-1,2-ethylenediyl)bisphenol
161. Phenol, 4,4'-(1,2-diethyl-1,2-ethylenediyl)bis-, (z)-
162. Trans-diethyl Stilbestrol
163. Diethylstilbestrol [nonsteroidal Oestrogens]
164. Prestwick2_000756
165. Prestwick3_000756
166. Spectrum5_000799
167. 4, 2,2'-diethyl-
168. Schembl9223
169. Bspbio_000772
170. Bspbio_002201
171. 4,4'-stilbenediol, Alpha,alpha'-diethyl-, (z)-
172. Mls000028447
173. Mls002174252
174. Mls002222298
175. Bidd:er0159
176. Spectrum1500244
177. Diethylstilbestrol [mi]
178. 4-[(3e)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol
179. Bpbio1_000850
180. Gtpl2801
181. Trans-.alpha.,4'-stilbenediol
182. Zinc1290
183. 4, .alpha.,.alpha.-diethyl-
184. Diethylstilbestrol [inn]
185. 4-[(e)-1-ethyl-2-(4-hydroxyphenyl)but-1-enyl]phenol
186. Dtxsid3020465
187. Regid_for_cid_448537
188. Bdbm20625
189. Chebi:92795
190. Diethylstilbestrol [hsdb]
191. Diethylstilbestrol [iarc]
192. Hms501j21
193. Rglykwwbqgjzgm-islyrvaysa-
194. 4, .alpha.,.alpha.'-diethyl-
195. Diethylstilbestrol [vandf]
196. Nsc3070
197. Wln: Qr Dy2&uy2&r Dq
198. 3,4'-dihydroxyphenyl)hex-3-ene
199. Diethylstilbestrol [mart.]
200. Hms1570g14
201. Hms1920g08
202. Hms2090c14
203. Hms2091m18
204. Hms2097g14
205. Hms2232n11
206. Hms3650a09
207. Hms3714g14
208. Pharmakon1600-01500244
209. 3-hexene,4-bis(p-hydroxyphenyl)-
210. Diethylstilbestrol [usp-rs]
211. Diethylstilbestrol [who-dd]
212. Nsc 3070
213. Tox21_202407
214. Tox21_300526
215. Ccg-38961
216. Diethylstilbestrol, >=99% (hplc)
217. Nsc756736
218. S1859
219. Stk366318
220. 3,4-bis(4-hydroxyphenyl)-3-hexene
221. Akos005111142
222. Diethylstilbestrol (diethylstilbestr
223. 4,2-diethyl-1,2-ethenediyl)bisphenol
224. Db00255
225. Nsc-756736
226. Diethylstilbestrol [orange Book]
227. Idi1_000519
228. Diethylstilbestrol [ep Monograph]
229. Ncgc00090749-01
230. Ncgc00090749-02
231. Ncgc00090749-03
232. Ncgc00090749-05
233. Ncgc00090749-06
234. Ncgc00090749-07
235. Ncgc00090749-08
236. Ncgc00090749-09
237. Ncgc00090749-15
238. Ncgc00254539-01
239. Ncgc00259956-01
240. 4, .alpha.,.alpha.'-diethyl-, (e)-
241. As-13377
242. Diethylstilbestrol [usp Monograph]
243. Hy-14598
244. Ls-14649
245. Bovine Muscle (diethylstilbestrol Blank)
246. Diethylstilbestrol,mixture Of Cis And Trans
247. Sbi-0051346.p003
248. 3,4-bis(p-hydroxyphenyl)-3-hexene, Trans
249. Cs-0369240
250. D0526
251. Sw197137-4
252. .alpha.,.alpha.'-diethyl-4,4'-stilbenediol
253. 3,4-(di-4-hydroxyphenyl)hex-3-ene, (e)-
254. 3-hexene,3,4-bis(p-hydroxyphenyl)-, (e)-
255. 4,4'-dihydroxy-.alpha.,.beta.-diethylstilbene
256. C07620
257. D00577
258. D81873
259. 002d373
260. A831077
261. Diethylstilbestrol, Mixture Of Cis And Trans, 97%
262. Q423989
263. Sr-01000745070-3
264. Sr-01000745070-4
265. Sr-01000745070-5
266. Sr-01000745070-9
267. Trans-.alpha.,.alpha.'-diethyl-4,4'-stilbenediol
268. Trans-.alpha.,.alpha.'-diethyl-4-4'-stilbenediol
269. 4,4'-(1,2-diethyl-1,2-ethenediyl)bisphenol, Trans
270. 4,4'-stilbenediol, .alpha.,.alpha.-diethyl-, (e)-
271. 4,4'[(1e)-1,2-diethyl-1,2-ethenediyl]bis-phenol
272. Brd-k17084514-001-01-7
273. Brd-k45330754-001-09-2
274. Diethylstilbestrol, Vetranal(tm), Analytical Standard
275. Phenol,4'-(1,2-diethyl-1,2-ethenediyl)bis-, (e)-
276. Diethylstilbestrol, European Pharmacopoeia (ep) Reference Standard
277. Diethylstilbestrol, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 268.3 g/mol |
|---|---|
| Molecular Formula | C18H20O2 |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 268.146329876 g/mol |
| Monoisotopic Mass | 268.146329876 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 286 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...DES also have been used in the treatment of prostate cancer to reduce testicular androgen production secondary to inhibition of LH release from the pituitary.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1421
... Two major uses are as a component of combination oral contraceptives and for hormone replacement therapy in postmenopausal women. /Estrogens/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1421
Chemotherapeutic agents useful in neoplastic disease /of the/ breast, prostate /from table/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1229
Antineoplastic Agents, Hormonal; Carcinogens; Contraceptives, Postcoital, Synthetic; Estrogens, Non-Steroidal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
For more Therapeutic Uses (Complete) data for DIETHYLSTILBESTROL (14 total), please visit the HSDB record page.
Nausea & vomiting are an initial reaction...Fullness & tenderness of the breast & edema...Severe migraine in some...Reactivate or exacerbate endometriosis and its attendant pain. /Estrogens/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1421
DIETHYLSTILBESTROL TAKEN DURING PREGNANCY HAS BEEN SHOWN TO BE CAUSALLY ASSOC WITH INCR IN VAGINAL AND CERVICAL CLEAR-CELL ADENOCARCINOMA IN DAUGHTERS, PRIMARILY IN THOSE BETWEEN THE AGES OF 10 AND 30 YR. THE RISK APPEARS TO BE IN THE ORDER OF 0.14-1.4/1000 EXPOSED DAUGHTERS UP TO THE AGE OF 24 YR.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 209 (1977)
VET: TOXIC EFFECTS INCLUDE THROMBOCYTOPENIA, GYNECOMASTIA, AND FLUID RETENTION.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 796
... FIVE PATIENTS HAD SYMPTOMS OF PRESBYOPIA ASSOCIATED WITH USE OF DIETHYLSTILBESTROL & ... THESE SYMPTOMS ABATED ON DISCONTINUANCE OF DRUG.
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 941
For more Drug Warnings (Complete) data for DIETHYLSTILBESTROL (24 total), please visit the HSDB record page.
Used in the treatment of prostate cancer. Previously used in the prevention of miscarriage or premature delivery in pregnant women prone to miscarriage or premature delivery.
Diethylstilbestrol is a synthetic estrogen that was developed to supplement a woman's natural estrogen production. In 1971, the Food and Drug Administration (FDA) issued a Drug Bulletin advising physicians to stop prescribing DES to pregnant women because it was linked to a rare vaginal cancer in female offspring.
Estrogens, Non-Steroidal
Non-steroidal compounds with estrogenic activity. (See all compounds classified as Estrogens, Non-Steroidal.)
Carcinogens
Substances that increase the risk of NEOPLASMS in humans or animals. Both genotoxic chemicals, which affect DNA directly, and nongenotoxic chemicals, which induce neoplasms by other mechanism, are included. (See all compounds classified as Carcinogens.)
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03C - Estrogens
G03CB - Synthetic estrogens, plain
G03CB02 - Diethylstilbestrol
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03C - Estrogens
G03CC - Estrogens, combinations with other drugs
G03CC05 - Diethylstilbestrol
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02A - Hormones and related agents
L02AA - Estrogens
L02AA01 - Diethylstilbestrol
ONLY TRACES ... COULD BE FOUND IN TISSUES 24 HR AFTER ADMIN TO SHEEP & GOATS.
Garner's Veterinary Toxicology. 3rd ed., rev. by E.G.C. Clarke and M.L. Clarke. Baltimore: Williams and Wilkins, 1967., p. 179
... SMALL AMT EXCRETED UNCHANGED. STILBESTROL LABELLED WITH (14)C IN TWO METHYLENE GROUPS, INJECTED IN SMALL DOSES INTO RATS, IS MOSTLY EXCRETED IN BILE ... ONLY 5% OF DOSE IS EXCRETED IN URINE. NO (14)CO2 IS EXCRETED IN EXPIRED AIR, SO PRESUMABLY MOLECULE IS STABLE.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 170
DES is readily absorbed from the GI tract following oral administration. The drug is slowly inactivated in the liver and excreted in urine and feces, principally as the glucuronide.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2693
Hepatic.
IN SMALL DOSES, ABOUT 70% IS CONJUGATED WITH GLUCURONIC ACID AT ONE OF TWO HYDROXYL GROUPS, VERY LITTLE SULFATE CONJUGATION OCCURS, & ONLY SMALL AMT ARE EXCRETED UNCHANGED.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 170
MAJOR METABOLITES OF DES IN SEVERAL SPECIES (RAT, MOUSE, HAMSTER, PRIMATES) ARE DIENOESTROL AND OMEGA-HYDROXYDIENOESTROL ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 197 (1979)
OXIDATIVE METABOLISM OF DES WAS MEASURED IN MALE & FEMALE GENITAL TRACTS OF FETAL MOUSE IN ORGAN CULTURE. MAJOR OXIDATIVE METABOLITE WAS Z,Z-DIENESTROL, WHOSE FORMATION APPEARED TO BE TIME DEPENDENT IN ISOLATED FETAL GENITAL TRACT OF BOTH SEXES. IN ADDN, FETAL GENITAL TRACTS WERE CAPABLE OF O-METHYLATION OF DES. A NEW METABOLITE, 4'-O-METHYL-DES, WAS FORMED IN FETAL GENITAL TISSUES BUT NOT IN LIVER CULTURES. CONJUGATION OF DES OCCURRED EXTENSIVELY IN FETAL LIVER & PLACENTA BUT NOT IN FETAL GENITAL TISSUES.
PMID:6861692 MAYDL R ET AL; ENDOCRINOLOGY 113 (1): 146 (1983)
Microsomes were prepared from livers of untreated male hamsters (8 wk old) by differential centrifugation. Microsome-mediated reactions were carried out using 10 to 250 uM diethylstilbestrol (DES) with (0.5 to 2.0 mM) cumene hydroperoxide or 100 uM DES with 1 to 5 mM nicotinamide adenine dinucleotide phosphate. Diethylstilbestrol-4',4''-quinone formation by fetal liver homogenate was carried out by incubating 100 uM DES, 1.5 mM cumene hydroperoxide and fetal homogenate (4 mg/ml protein) for 10 min. In vitro, the time dependent formation of diethylstilbestrol-4',4''-quinone as a function of microsomal protein, cofactor or substrate concn was demonstrated. Quinone formation was time dependent and increased in linear fashion for up to 10 min, then remained at a plateau level when incubation times were further increased. Diethylstilbestrol-4',4''-quinone was also formed by fetal liver homogenate. The microsome mediated oxidation of DES to quinone was inhibited 93% by 500 uM 2(3-t-butyl-4-hydroxyanisole), 96% by 500 uM N,N,N',N'-tetramethyl-p-phenylenediamine, 83% by 500 uM n-octylamine, 97% by 500 uM potassium cyanide, and 6% by 1 mM cyclohexene oxide. In microsomal incubations with nicotinamide-adenine dinucleotide phosphate, quinone formation was below detection limits (< 0.005 nmol/mg protein/min).
PMID:2736717 Roy D, Liehr JG; Carcinogenesis 10 (7): 1241-5 (1989)
For more Metabolism/Metabolites (Complete) data for DIETHYLSTILBESTROL (7 total), please visit the HSDB record page.
Estrogens diffuse into their target cells and interact with a protein receptor, the estrogen receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. The effect of Estrogen binding their receptors causes downstream increases the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. The combination of an estrogen with a progestin suppresses the hypothalamic-pituitary system, decreasing the secretion of gonadotropin-releasing hormone (GnRH).
The precise mechanism(s) of action of DES as a postcoital contraceptive is not fully understood; however, the drug appears to inhibit nidation (implantation) of the fertilized ovum in the endometrium when administered within 72 hours following coitus. The postcoital contraceptive activity of the drug may involve effects mediated via decreased concentrations of circulating progesterone, effects on tubal motility resulting in accelerated passage of the ovum into the uterus, and inhibition of synthesis of carbonic anhydrase in the endometrium.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2693
... DES ... inhibited the postcastration rise in plasma FSH amd LH levels ... . In addition, DES stimulated large increases in prolactin secretion ...
Thomas, J.A., K.S. Korach, J.A. McLachlan. Endocrine Toxicology. New York, NY: Raven Press, Ltd., 1985., p. 372
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?