
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
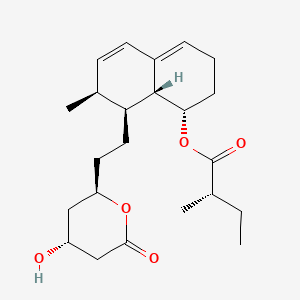

1. 6-demethylmevinolin
2. Compactin
3. Cs 500
4. Ml 236b
5. Ml-236b
6. Ml236b
1. 73573-88-3
2. Compactin
3. Ec 700-442-0
4. Ml-236b
5. Mevastatin [inn]
6. Mevastatina
7. Mevastatine
8. Mevastatinum
9. Mevastatinum [inn-latin]
10. Antibiotic Ml 236b
11. Cs 500
12. Ml 236 B
13. [(1s,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2s)-2-methylbutanoate
14. 1uqm1k0w9x
15. Mevastatin (compactin)
16. Chembl54440
17. Chebi:34848
18. Ml236b
19. Nsc-759322
20. (1s,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-7-methyl-8-(2-((2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthyl (s)-2-methylbutyrate
21. Compactin (penicillium)
22. Mevastatine [inn-french]
23. Mevastatina [inn-spanish]
24. (1s,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (s)-2-methylbutanoate
25. (s)-((1s,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl) 2-methylbutanoate
26. Butanoic Acid, 2-methyl-, (1s,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-[(2r,4r)-tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl]ethyl]-1-naphthalenyl Ester, (2s)-
27. Smr000336944
28. Ccris 4505
29. Unii-1uqm1k0w9x
30. Kompaktin
31. Ncgc00095942-01
32. Mevastatin- Bio-x
33. Mfcd05662341
34. Mevastatin [mi]
35. Mevastatin [jan]
36. Mevastatin [mart.]
37. Schembl1116
38. Dsstox_cid_20684
39. Dsstox_rid_79540
40. Mevastatin [usp-rs]
41. Dsstox_gsid_40684
42. Lopac0_000754
43. Mls000721804
44. Mls000759452
45. Mls001424284
46. Mls002207227
47. Gtpl3031
48. Dtxsid4040684
49. Mevastatin, >=96% (hplc)
50. Hms2052p07
51. Hms2089d10
52. Hms2232n09
53. Hms3262g10
54. Hms3268a19
55. Hms3412h15
56. Hms3676h15
57. Hms3713b06
58. Zinc3833876
59. Tox21_111540
60. Tox21_500754
61. Bdbm50011036
62. Cs-500
63. Nsc779705
64. S4223
65. Akos015994712
66. Mevastatin, >=95% (hplc), Powder
67. Ccg-101174
68. Cs-1234
69. Db06693
70. Ks-1085
71. Lp00754
72. Nc00424
73. Nsc 759322
74. Nsc-779705
75. Sdccgsbi-0050732.p002
76. 7-(1,2,6,7,8,8a-hexahydro-2-methyl-8-(2-methylbutyryloxy)naphthyl)-3-hydroxyheptan-5-olide
77. Smp1_000077
78. Ncgc00025202-04
79. Ncgc00261439-01
80. (1s,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl)ethyl)-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl(2s)-2-methylbutanoate
81. Bm164666
82. Butanoic Acid, 2-methyl-, (1s,7s,8s,8ar)-1,2,3,7,8,8a-hexahydro-7-methyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (2s)-
83. Butanoic Acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-7-methyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl)ethyl)-1-naphthalenyl Ester, (1s-(1-alpha(r*),7-beta,8-beta(2s*,4s*),8a-beta))-
84. Hy-17408
85. Mevastatin 100 Microg/ml In Acetonitrile
86. Cas-73573-88-3
87. Lovastatin Impurity A [ep Impurity]
88. B1788
89. Eu-0100754
90. M2275
91. H11997
92. M 2537
93. Ab00588266-06
94. Ab00588266-08
95. Ab00588266_09
96. 573m883
97. A837861
98. Q414407
99. Sr-01000729493
100. L-637312
101. Sr-01000729493-4
102. Brd-k94441233-001-03-1
103. Brd-k94441233-001-17-1
104. (1s,7s,8s,8ar)-8-{2-[(2r,4r)-4-hydroxy-6-oxotetrahydro-2h-pyran-2-yl]ethyl}-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2s)-2-methylbutanoate
105. (s)-((1s,7s,8s,8ar)-8-(2-((2r,4r)-4-hydroxy-6-oxo-tetrahydro-2h-pyran-2-yl)ethyl)-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl) 2-methylbutanoate
106. (s)-2-methyl-butyric Acid (1s,7s,8s,8ar)-8-[2-((2r,4r)-4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-7-methyl-1,2,3,7,8,8a-hexahydro-naphthalen-1-yl Ester
107. 2-methyl-butyric Acid 8-[2-(4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-7-methyl-1,2,3,7,8,8a-hexahydro-naphthalen-1-yl Ester
108. 2-methyl-butyric Acid 8-[2-(4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-7-methyl-1,2,3,7,8,8a-hexahydro-naphthalen-1-yl Ester((+)-compactin)
109. 2-methyl-butyric Acid 8-[2-(4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-7-methyl-1,2,3,7,8,8a-hexahydro-naphthalen-1-yl Ester(compactin)
| Molecular Weight | 390.5 g/mol |
|---|---|
| Molecular Formula | C23H34O5 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 390.24062418 g/mol |
| Monoisotopic Mass | 390.24062418 g/mol |
| Topological Polar Surface Area | 72.8 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 637 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Not used therapeutically due to its many side effects.
The primary cause of cardiovascular disease is atherosclerotic plaque formation. Mevastatin lowers hepatic production of cholesterol to reduce the risk of cardiovascular disease. Mevastatin competitively inhibits HMG-CoA reductase. This inhibition prevents the rate limiting step in cholesterol synthesis. Decreased hepatic cholesterol levels causes increased uptake of low density lipoprotein (LDL) cholesterol and reduces cholesterol levels in the circulation.
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Compounds that inhibit HYDROXYMETHYLGLUTARYL COA REDUCTASES. They have been shown to directly lower CHOLESTEROL synthesis. (See all compounds classified as Hydroxymethylglutaryl-CoA Reductase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Mevastatin is structurally similar to the HMG, a substituent of the endogenous substrate of HMG-CoA reductase. Mevastatin is a prodrug that is activated in vivo via hydrolysis of the lactone ring. The hydrolyzed lactone ring mimics the tetrahedral intermediate produced by the reductase allowing the agent to bind with 10,000 times greater affinity than its natural substrate. The bicyclic portion of mevastatin binds to the coenzyme A portion of the active site.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?